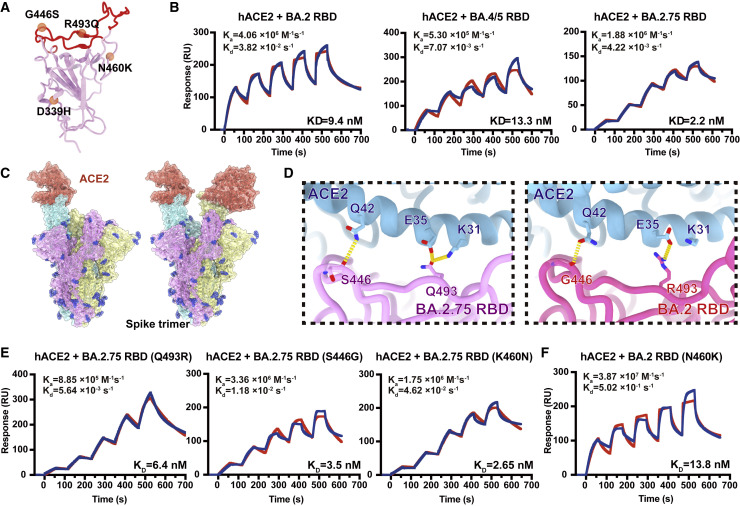
Figure 2.
BA.2.75 exhibited enhanced human ACE2 binding
(A) Position distribution of important amino acids in BA.2.75 RBD. The mutated residues relative to BA.2 RBD are marked as orange globules. RBM is colored in red.
(B) Binding affinities of RBDs of BA.2, BA.4/5, and BA.2.75 subvariants to hACE2 measured by SPR.
(C) Overall structure of the BA.2.75 S-trimer in complex with hACE2. Three copies of S monomer are colored in yellow, cyan, and magenta, respectively. The hACE2 molecules bound to RBD are colored in orange.
(D) Changes at the interfaces between BA.2.75 RBD (left) and BA.2 RBD (PDB: 7ZF7, right) with hACE2. Key mutated residues are shown as sticks and hydrogen bonds are shown as yellow dash lines.
(E) Binding affinity of hACE2 with BA.2.75 RBD with single substitution Q493R, S446G, and K460N measured by SPR.
(F) Binding affinity of hACE2 with BA.2+N460K RBD measured by SPR.
See also Figure S1.