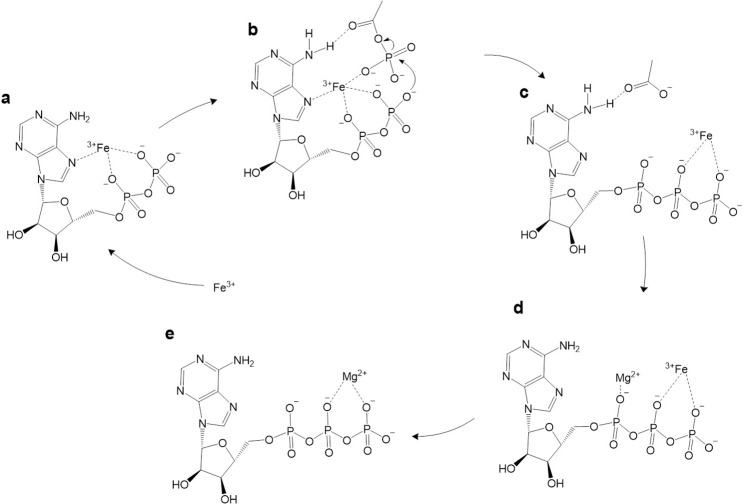
Fig 7. Potential mechanism of ADP phosphorylation in water.
Fe3+, stabilised by the N7 group on adenine, interacts with the dianion of ADP, lowering the pKa of the outermost OH group and enhancing its nucleophilicity (a). Fe3+ interacts with the oxygens of a molecule of the surrounding AcP, bringing it close enough to facilitate the phosphate transfer (b). This interaction might be stabilised by further interactions between the N6 amino group and the carboxylate oxygen on AcP (b). Note that we only depict interactions between the Fe3+ and moieties on the ADP, adenosine ring, and AcP that have been established by our experiments and MD simulations, and this is not intended as a full depiction of the coordination sphere; for clarity, we do not include any interactions with water, which would certainly be part of the coordination sphere. Fe3+ is then likely to move from Pα to the Pβ and Pγ of ATP, although this is uncertain (c), and ultimately abandons the ATP chelated by acetate groups facilitated by the favourable association of Mg2+ as suggested by the reaction kinetics (d). Fe3+ is then available to catalyse another phosphorylation of ADP (e). AcP, acetyl phosphate; MD, molecular dynamic.