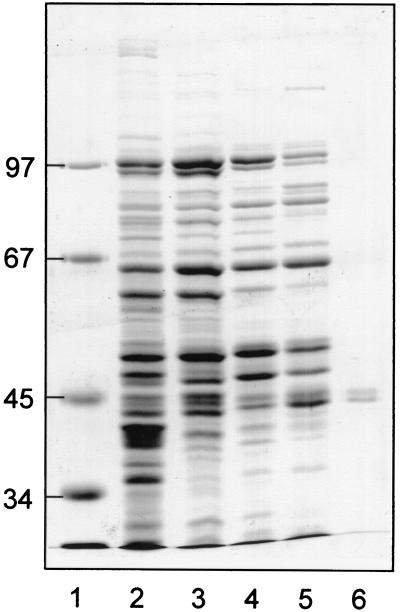
FIG. 3.
SDS-PAGE of active fractions during purification of succinyl-CoA:benzylsuccinate CoA-transferase. A Coomassie-stained gel is shown, in which the pooled fractions of the purification were analyzed. Low polyacrylamide concentrations (8% [wt/vol]) were used to separate the two CoA-transferase subunits. The purified enzyme was checked for the absence of contaminating proteins smaller than 30 kDa, which migrated in the front in these gels. Lanes: 1, protein standards; 2, crude extract (16 μg of protein); 3, DEAE-Sepharose fraction (12 μg of protein); 4, hydroxyapatite fraction (12 μg of protein); 5, Q-Sepharose fraction (12 μg of protein); 6, gel filtration fraction (1.5 μg of protein). The masses of standard proteins in kilodaltons are indicated on the left margin.