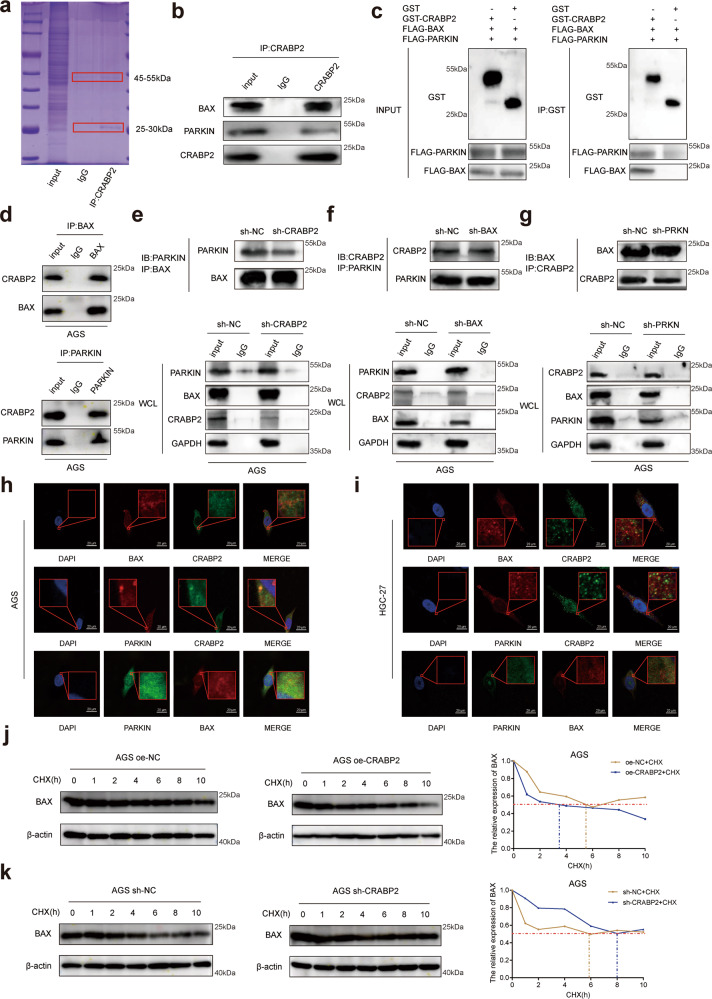
Fig. 3. CRABP2 expedited the binding ability of BAX and PARKIN in GC.
a Total proteins from Flag-CRABP2 plasmid-transfected AGS cells were separated via SDS–PAGE. PARKIN and BAX were identified by LC/LC–MS in the CRABP2 protein complex. b Mutual interactions of BAX/PARKIN and Flag-CRABP2 were verified by the Co-IP assay. c The BAX and PARKIN proteins were pulled down by GST-CRABP2 fusion protein-bound beads by SDS–PAGE analysis. d The interaction between BAX and CRABP2 and between PARKIN and CRABP2 was verified by co-IP assay and western blotting. e After interference with CRABP2 in AGS cells, the binding ability of BAX to PARKIN decreased. f After BAX interference in AGS cells, the binding ability of CRABP2 to PARKIN was not affected. g After interference with PARKIN in AGS cells, the binding ability of CRABP2 to PARKIN was not affected. The immunofluorescence assay showed the subcellular localizations (red frame) of CRABP2/BAX/PARKIN in AGS (h) and HGC-27 (i) cells. AGS cells overexpressing (j) or knocking down (k) CRABP2 were treated with cycloheximide (CHX) for 0, 2, 4, 6, 8, or 10 h. The expression of Bax was detected by western blotting. All experiments were performed in three replicates. *P < 0.05, **P < 0.01, ***P < 0.001. (IB immunoblotting, IP immunoprecipitation, WCL whole cell lysates).