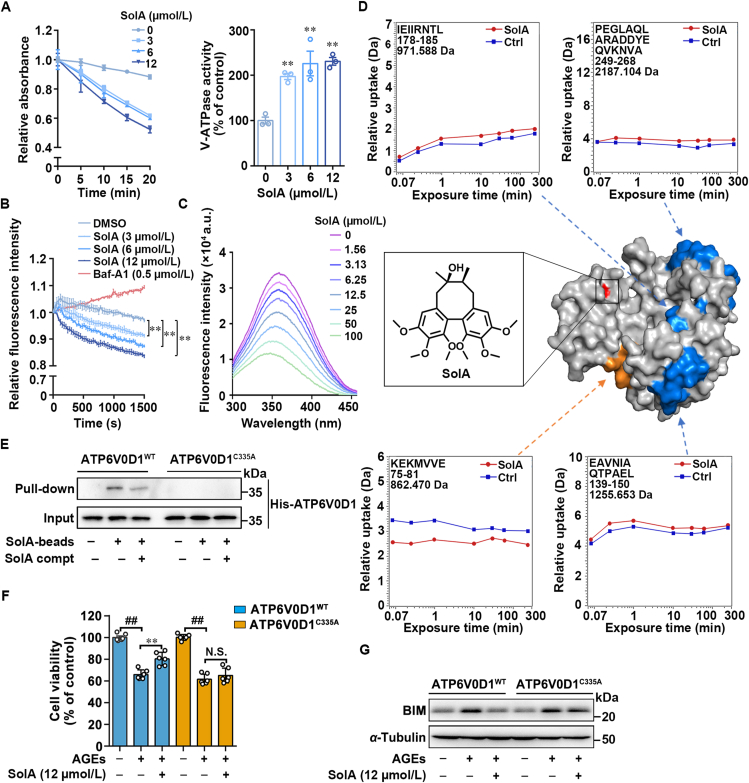
Figure 5.
Cys335 serves as a pharmacological allosteric site of ATP6V0D1. (A) SolA promoted V-ATPase activity in a concentration-dependent manner (n = 3). (B) SolA increased the rate of H+ pumping in a concentration-dependent manner (n = 3). (C) SolA mediated ATP6V0D1 conformational change by tryptophan fluorescence quenching analysis. (D) SolA allosterically regulated ATP6V0D1 conformation by hydrogen-deuterium exchange mass spectrometry (HDXMS). SolA binding site is shown in red. Peptides with increased deuterium uptake ratio after SolA treatment are highlighted in orange, and peptides with decreased deuterium uptake ratio were highlighted in blue (PDB: 6wlw). (E) Pull-down analysis of SolA with ATP6V0D1WT or ATP6V0D1C335A. (F) ATP6V0D1 C335A mutation inhibited SolA (12 μmol/L)-mediated protection in PC12 cells by MTT assay (n = 6). (G) SolA (12 μmol/L)-induced BIM degradation was blocked by ATP6V0D1 C335A mutation. Data are expressed as the mean ± SEM. ##P < 0.01 vs. control group, ∗∗P < 0.01 vs. AGEs group. N.S. not significant.