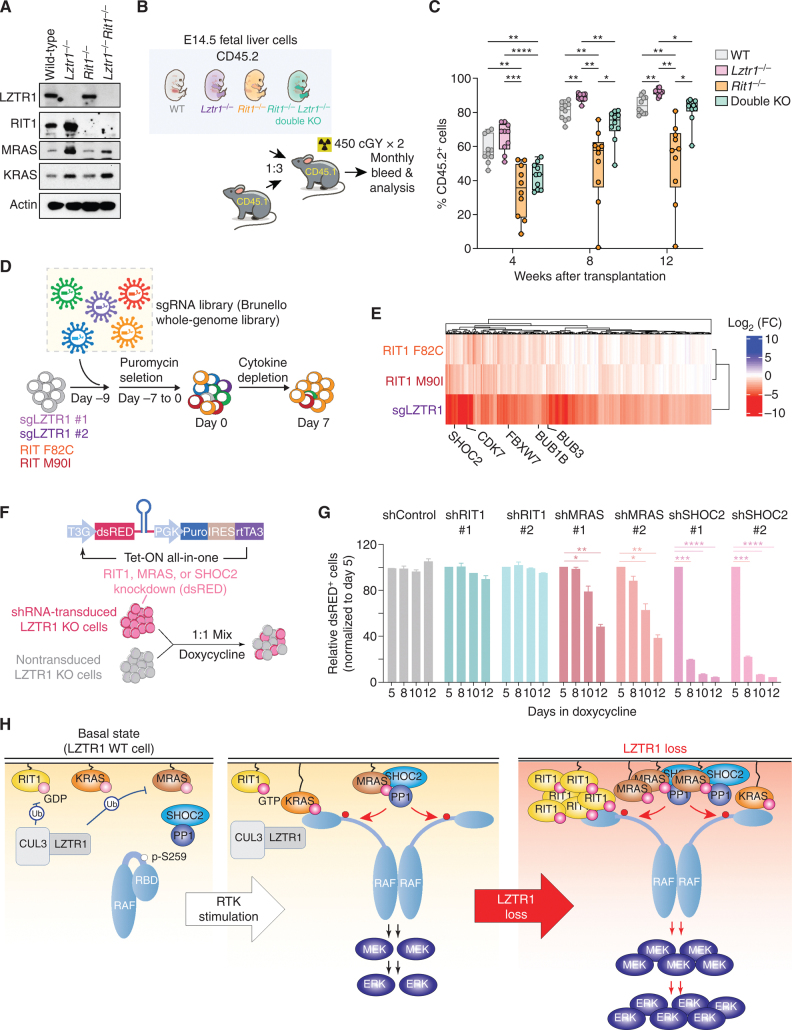
Figure 5.
Lztr1-null cells depend on multiple RAS GTPases. A, Western blot of E14.5 fetal liver cells from mice with germline deletion of Lztr1, Rit1, or both Lztr1 and Rit1. B, Schema of experiments evaluating effects of Lztr1 or Rit1 deletion, alone or together, on fetal hematopoietic cells in vivo. C, Peripheral blood chimerism of the experiment in B. n = 10/group. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.001. For box and whiskers plots, bar indicates median; box edges, first and third quartile values; and whisker edges, minimum and maximum values. D, Schema of positive enrichment custom CRISPR–Cas9 pooled lentiviral screen to identify genes required for cytokine-independent growth following LZTR1 deletion or expression of RIT1 mutations in TF-1 cells. E, Heat map of sgRNAs depleted in RIT1 F82C, RIT1 M90I, or LZTR1 KO TF-1 cells following cytokine depletion. Log2 fold change (FC) is shown. F, Schema of growth competition assay to evaluate effects of RIT1, MRAS, or SHOC2 suppression on LZTR1 WT or KO cells. G, Relative ratio of shRNA-expressing (dsRED+) LZTR1 KO TF-1 cells following culture in doxycycline and removal of GM-CSF. Doxycycline induces expression of dsRED simultaneously with expression of shRNAs targeting Renilla (“shRen,” a negative control), RIT1, MRAS, or SHOC2. H, Schema of the effects of LZTR1 loss on signaling in hematopoietic cells. At baseline, LZTR1 restrains the abundance of multiple RAS GTPases, including RIT1, KRAS, and MRAS (left). Upon receptor tyrosine kinase (RTK) stimulation, RIT1, KRAS, and MRAS exchange GDP for GTP and enable RAF activation of MEK and ERK (middle box). LZTR1 depletion results in the accumulation of RIT1, KRAS, and MRAS, and the resultant cytokine hypersensitivity and transformation of LZTR1-null cells requires MRAS–SHOC2–PP1 activity.