Figure 1. Patient Disposition and Analysis Populations.
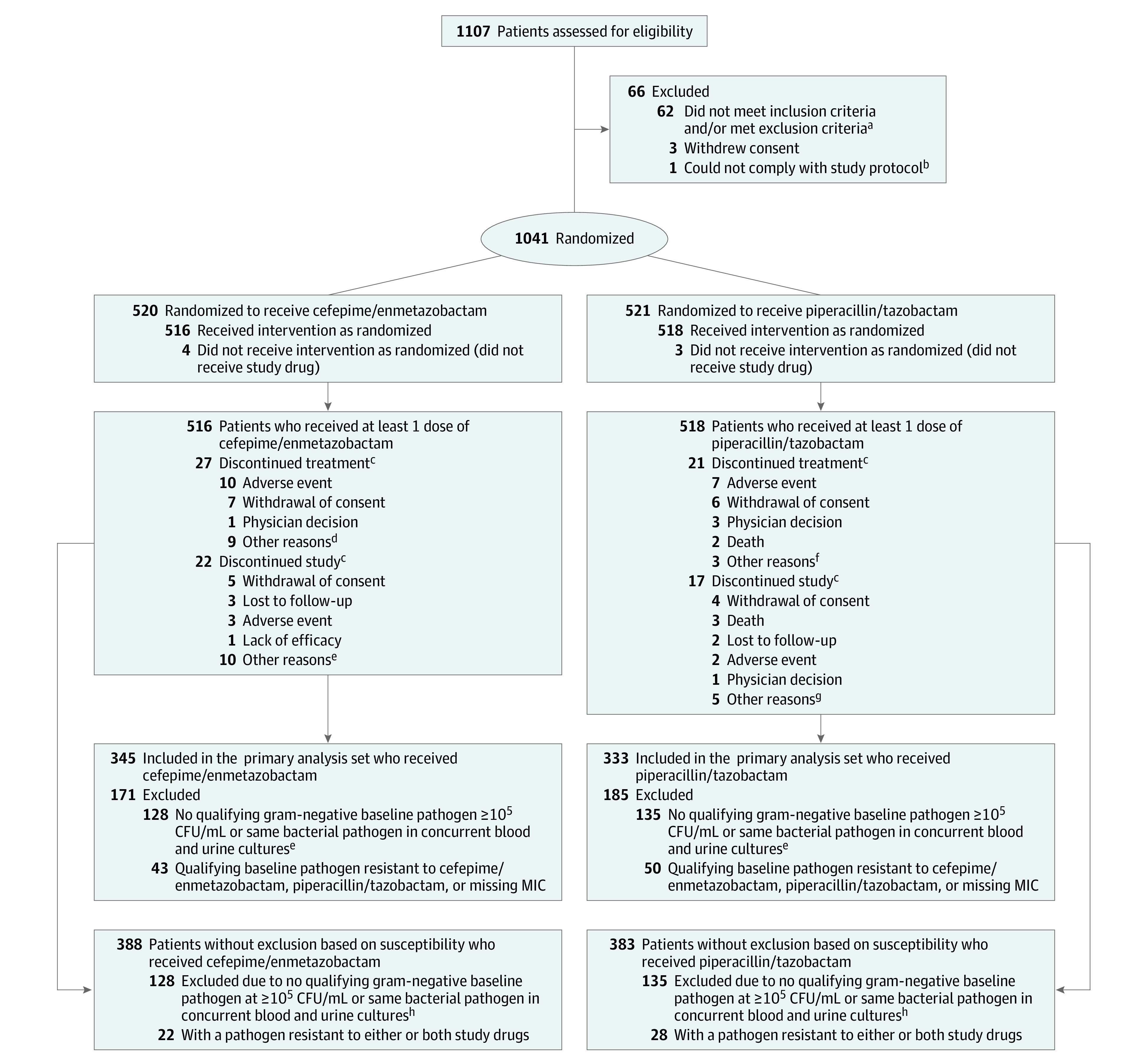
Analysis populations referenced in this study are described in the Methods section. Other study populations are defined in eTable 1 in Supplement 3.
aThe inclusion and exclusion criteria are listed in the eBox in Supplement 3.
bIntravenous drug administration was not feasible in this patient and therefore the patient was not able to comply with the protocol.
cA patient could discontinue treatment but remain in the study, discontinue treatment but discontinue study at a later date, or discontinue treatment and discontinue study at the same time.
dThree patients refused continuation of study treatment; 1 patient did not have clinical improvement; 1 patient was deemed resistant to cefepime; 1 patient had an estimated glomerular filtration rate drop below 30 mL/min/1.73 m2 on day 2; 1 patient did not receive last dose on day 7 by error; in 1 patient treatment could not be continued due to lack of investigational product; and 1 patient discharged themself early.
eNine patients did not return to site for assessment but were contacted and 1 with whom contact was lost.
fTwo patients refused continuation of study treatment and 1 patient had a negative confirmation result of urine culture.
gFour patients did not return to site for assessment but were contacted and 1 patient had a negative confirmation result of urine culture.
hIncludes 10 patients who had a baseline culture that was considered contaminated and 16 patients with a baseline gram-positive pathogen only.
