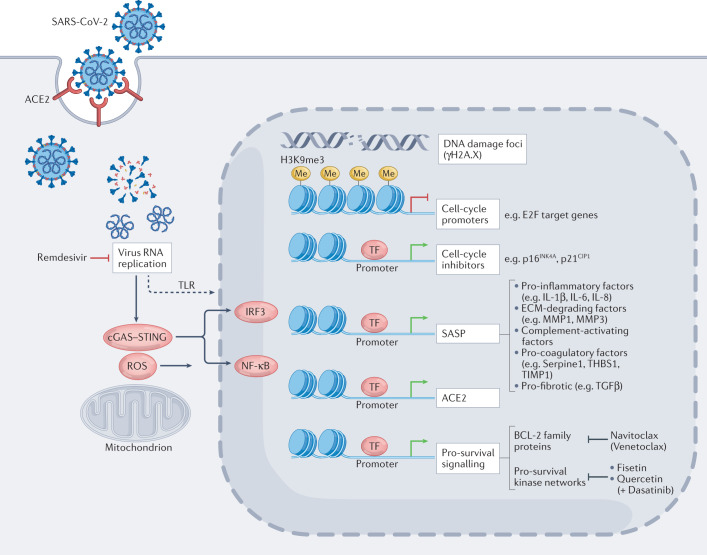
Fig. 1. Senescence-modulated host cell responses upon SARS-CoV-2 infection.
SARS-CoV-2 cellular entry induces senescence-typical transcriptional responses (see also Boxes 2 and 3), in both non-senescent or pre-existing senescent cells via viral replication stress, reactive oxygen species (ROS) production and cellular stress sensors such as toll-like receptor 3 (TLR3) and cGAS–STING signalling. Virus-induced senescence is characterized by senescence-associated, H3K9me3-positive heterochromatin foci (repressing, among others, S-phase-promoting E2F target genes), marks of DNA damage (that is, γH2A.X foci), the induction of cell-cycle inhibitors such as p16INK4a and p21CIP1, and pro-inflammatory, extracellular matrix-degrading, complement-activating, pro-coagulatory and pro-fibrotic senescence-associated secretory phenotype (SASP) factors (predominantly via IRF3, NF-κB and C/EBPβ transcription factors) that exacerbate tissue-destructive host immunity and potentiate the burden of senescent cells through paracrine and endocrine spread of senescence27–29,44 and may include elevated angiotensin-converting enzyme 2 (ACE2) expression27. Notably, pro-survival mechanisms selected for in stressed senescent cells, among them induction of anti-apoptotic BCL-2 family members and survival signalling-enhancing kinase networks involving, for example, SRC family kinases, are attractive targets for the selective pharmacological elimination of these cells; such agents are termed ‘senolytics’. ECM, extracellular matrix; MMP, matrix metalloproteinases; TF, transcription factors.