Fig 2.
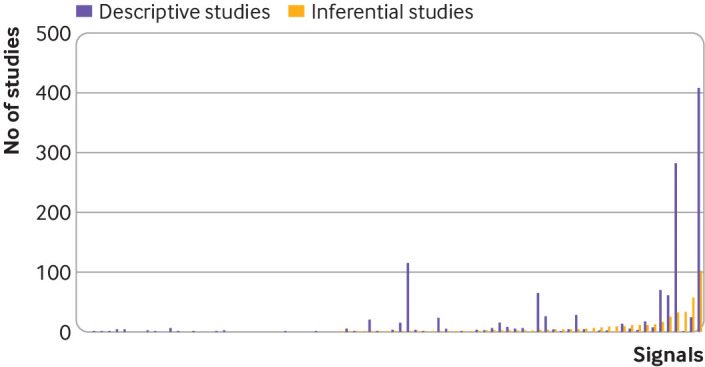
Number of published descriptive and inferential studies identified for each potential safety signal identified from the US Food and Drug Administration Adverse Event Reporting System in 2014-15. Each column pair represents one signal. Inferential studies=meta-analyses, prospective studies, and retrospective non-incidence or prevalence studies. Descriptive studies=retrospective incidence or prevalence studies and case series or case reports. Column pairs are ordered from left to right according to ascending number of inferential studies
