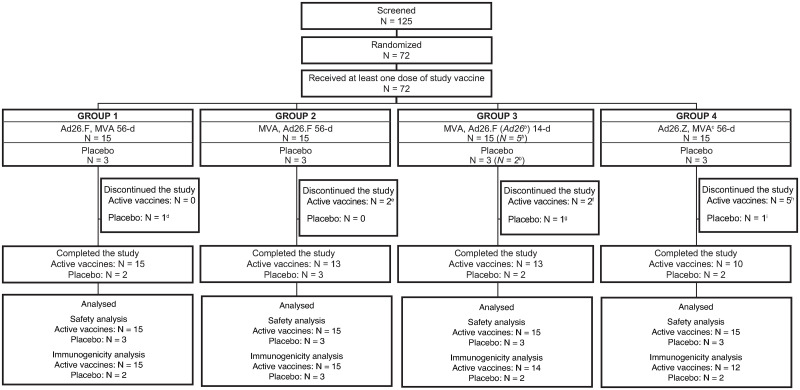
Fig 1. Participant dispositiona.
Ad26.F = Ad26.Filo; Ad26.Z = Ad26.ZEBOV; Inf U = infectious units; MVA = MVA-BN-Filo; N = number of participants. aA total of four participants had a major protocol deviation. In Group 1, one participant received disallowed concomitant therapy (bleomycin and cisplatin) during the post-dose 2 follow-up period. In Group 2, one participant did not have a Day 8 visit. In Group 4, one participant did not have a Day 8 visit, and another participant received a disallowed concomitant therapy (prednisone) and, as a result, discontinued the study prior to receiving the second vaccination. Participants were considered lost to follow-up after multiple unsuccessful contact attempts. bOnly a subset of Group 3 participants received a booster vaccination. cMVA-BN-Filo at a dose of 1x108 Inf U. dParticipant was unable to attend visits due to a family emergency. This participant had only received dose 1 vaccination. eParticipants were lost to follow-up. One of two participants had only received dose 1 vaccination. fOne participant was lost to follow-up and one participant suddenly relocated. One of two participants had only received dose 1 vaccination. gWithdrawal by the participant (no longer interested in participating). This participant had only received dose 1 vaccination. hWithdrawal by the participant (one participant was out of town and no longer interested in participating, one participant did not want to receive the second vaccination, one participant relocated, and one participant moved out of state) and one participant was lost to follow-up. One additional participant was unable to receive the second vaccination due to taking disallowed medication (prednisone) to treat an adverse event of left hip pain; this participant did not discontinue study participation. Three of five participants had only received dose 1 vaccination. iParticipant was lost to follow-up. This participant had only received dose 1 vaccination.