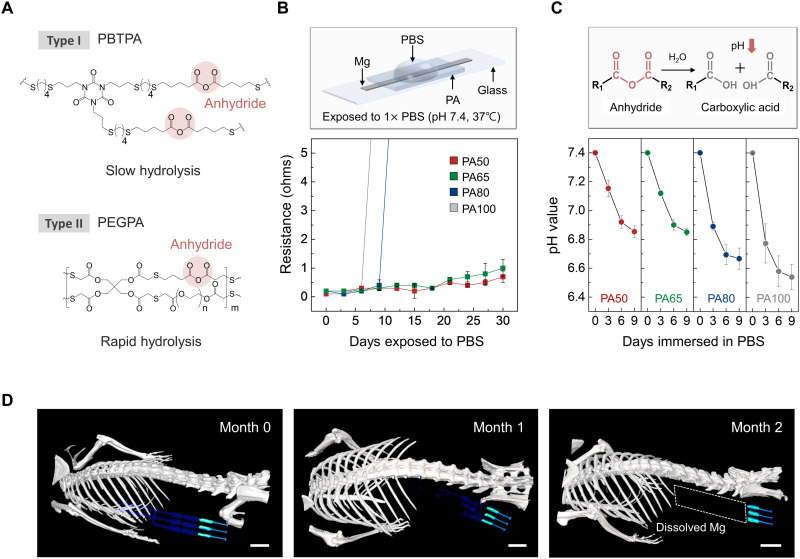
Fig. 3. Dissolution characteristics of an encapsulant studied in vitro and of a complete system studied in vivo.
(A) Chemical structure of the major components of the PA encapsulant. Synthesis occurs by photopolymerization of a liquid mixture of chemical precursors. Type I (PBTPA) and type II (PEGPA) have low and high rates of bioresorption, respectively, due to the hydrolysis of anhydride groups. (B) Changes in resistance of a Mg electrode (1 mm wide, 10 mm long, 50 μm thick) encapsulated with PA films with different formulations as a function of immersion time in 1× PBS (pH 7.4; 37°C). Independent samples, n = 4. Error bars, SD. (C) Time-dependent changes in pH of the PA films (5 mm wide, 5 mm long, 400 μm thick, 20 mg weight) during immersion in 1× PBS (pH 7.4; 37°C). Inset: Hydrolysis reaction of an anhydride group into two carboxylic acid groups. Independent samples, n = 4. Error bars, SD. (D) Three-dimensionally rendered CT images of mice collected over 2 months after implantation of a bioresorbable nerve stimulator. The images indicate the gradual disappearance of the Mg electrodes until they are no longer visible on month 2. Scale bars, 5 mm. Biologically independent mice, n = 4.