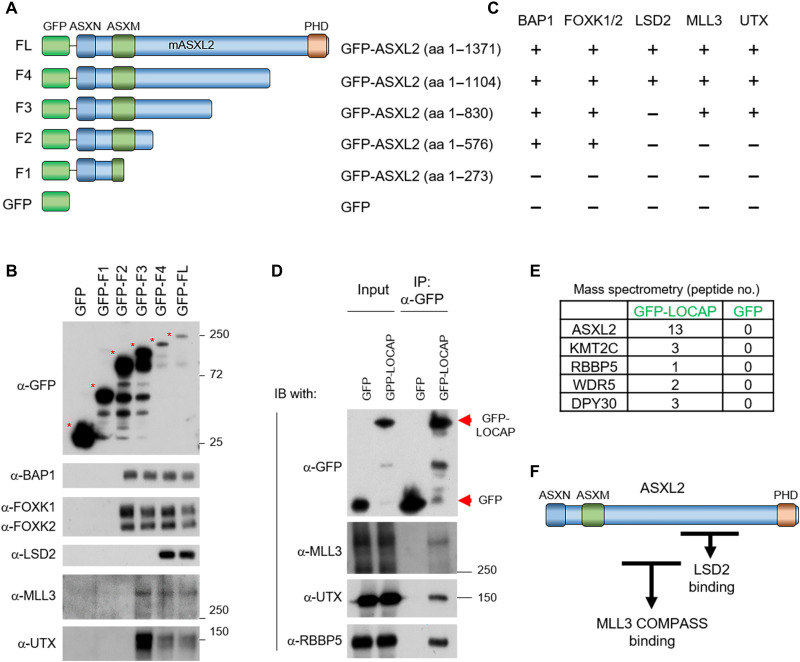
Fig. 2. Identification of LOCAP domain in ASXL2 that mediates MLL3/BAP1 interaction.
(A) A schematic diagram of full-length ASXL2 and its truncated derivatives. Both N-terminal domains (ASXN and ASXM) and a C-terminal domain (PHD) of ASXL2 are shown. (B) Plasmids expressing ASXL2 cDNA derivatives that correspond to each of the fragments shown in (A) were transfected into HEK293T cells for 24 hours. The GFP-tagged ASXL2 truncations were then purified and displayed interactions with BAP1, FOXK1/2, LSD2, MLL3, and UTX detected by Western blot. (C) The interaction between each ASXL2 fragments and BAP1, FOXK1/2, UTX, MLL3, and LSD2 is shown. The GFP-LOCAP domain fragment was expressed in HEK293T cells and then subjected to GFP purification from nuclear extracts. The protein-protein interaction between the ASXL2-LOCAP domain and MLL3 COMPASS was determined by Western blot (D) and mass spectrometry analysis (E); n = 3. (F) Protein interactome map of ASXL2 with MLL3 COMPASS [amino acids (aa) 576 to 830] and LSD2 (amino acids 830 to 1104) binding domains. MLL3 COMPASS binding domain is identified as the LOCAP domain.