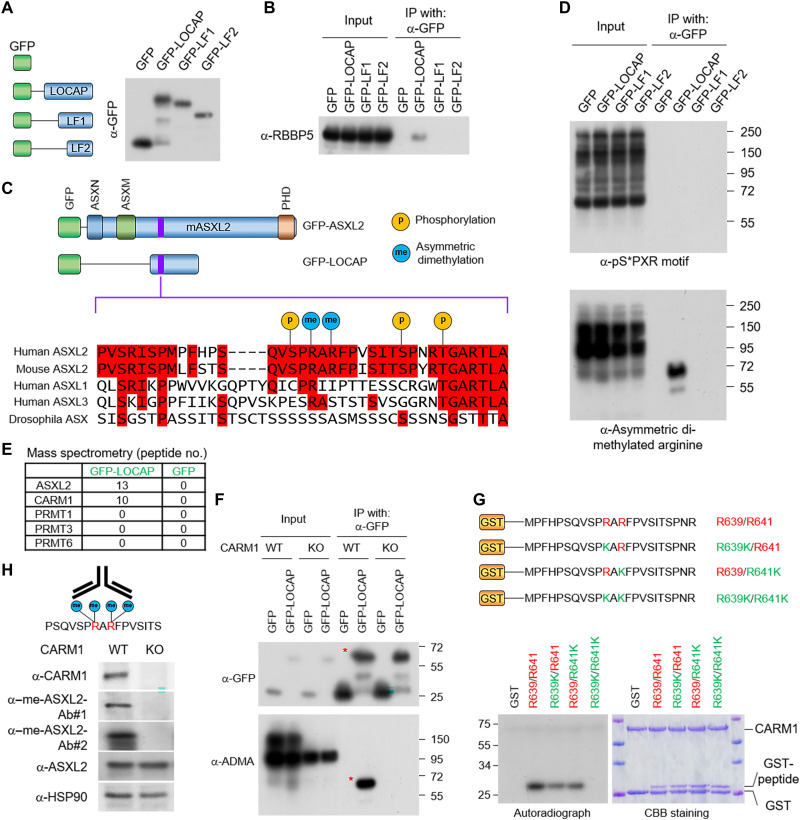
Fig. 3. Arginine methyltransferase CARM1 methylates ASXL2-LOCAP domain at R639/R641.
(A) A schematic diagram of the full-length ASXL2-LOCAP domain and its two truncated derivatives. The GFP-tagged LOCAP-FL, LOCAP-F1, and LOCAP-F2 were purified from HEK293T cells. (B) Western blot showing the interactions between each of the LOCAP domain truncations and RBBP5, one of the core subunits of MLL3 COMPASS. (C) PTMs at the LOCAP domain based on the PhosphoSitePlus database (www.phosphosite.org/homeAction.action). (D) IP of the GFP-tagged LOCAP derivatives coupled with Western blot detecting serine phosphorylation (via anti-serine phosphorylated antibody) (top) and arginine methylation (via anti-ADMA antibody) (bottom). The Western blot shows two arginine residues within LOCAP domain that are methylated in cells. (E) GFP-LOCAP was expressed in HEK293T cells and subjected to GFP purification from nuclear extracts and mass spectrometry analysis to identify which arginine methyltransferase is associated with ASXL2-LOCAP. The peptide number from all type II arginine methyltransferases is shown. (F) GFP-LOCAP was expressed in CARM1-WT/KO cells, and the methylation levels of LOCAP domain were determined by Western blot using anti-ADMA antibody. (G) Autoradiograph (left) and Coomassie brilliant blue staining (right) of in vitro methylated glutathione S-transferase (GST)–peptide fusion peptides by CARM1 in the presence of adenosyl-l-methionine, S-[methyl-3H] (SAM[3H]). Modifications to both arginine residues of ASXL2 (R639/R641) lead to loss of CARM1 protein-protein interaction. (H) Antigen peptide used for generation of methyl-specific antibody (left). Western blot analysis of the protein levels of CARM1, ASXL2, and me-ASXL2 (recognized by two antibodies) in CARM1-WT and KO cells (right). me-ASXL2, methylated ASXL2.