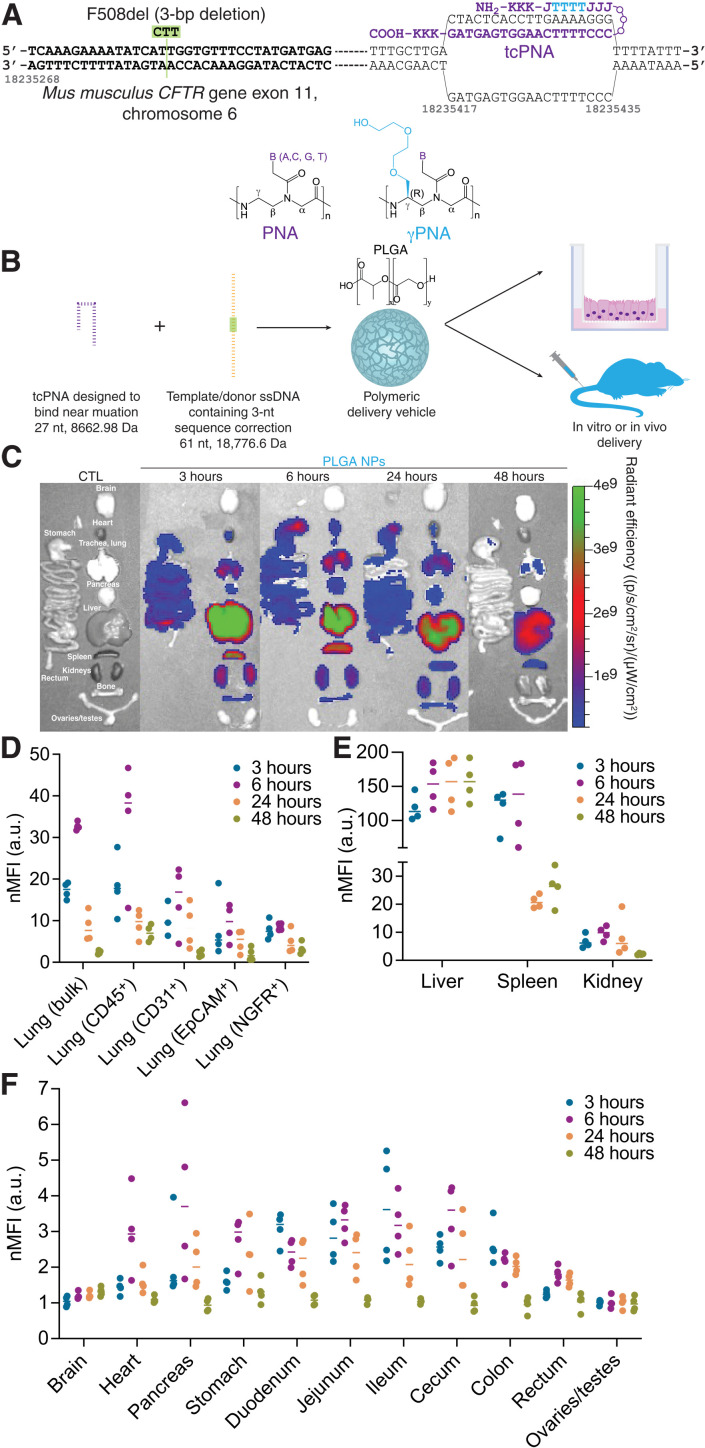
Fig. 1. PNA-based gene editing agents can be encapsulated into PLGA NPs, which exhibit accumulation in the lung and GI tract following systemic intravenous administration.
(A) Schematic of PNA design to correct F508del-CFTR, indicating the incorporation of γPNA monomers and the formation of the PNA/DNA/PNA triplex. (B) PNA and donor DNA in vitro and in vivo delivery strategy: Encapsulation into polymeric PLGA NPs. (C) Representative IVIS images indicating biodistribution of Cy5-conjugated PLGA NPs at 3, 6, 24, and 48 hours after intravenous administration in vivo compared to an untreated control animal (CTL). (D) Flow cytometry mean fluorescence intensity values normalized to untreated control animals (nMFI) for homogenized bulk lung and specific cell types (CD45+ macrophages, CD31+ endothelial cells, EpCAM+ epithelial cells, and NGFR+ basal cells) at 3, 6, 24, and 48 hours after intravenous administration of Cy5-conjugated PLGA NPs in vivo. Each dot represents data from one mouse. (E) Flow cytometry mean fluorescence intensity values normalized to untreated control animals (nMFI) for homogenized bulk liver, spleen, and kidney at 3, 6, 24, and 48 hours after intravenous administration of Cy5-conjugated PLGA NPs in vivo. Each dot represents data from one mouse. (F) Flow cytometry mean fluorescence intensity values normalized to untreated control animals (nMFI) for homogenized bulk brain, heart, pancreas, stomach, duodenum, jejunum, ileum, cecum, colon, rectum, and ovaries/testes at 3, 6, 24, and 48 hours after intravenous administration of Cy5-conjugated PLGA NPs in vivo. Each dot represents data from one mouse. a.u., arbitrary units.