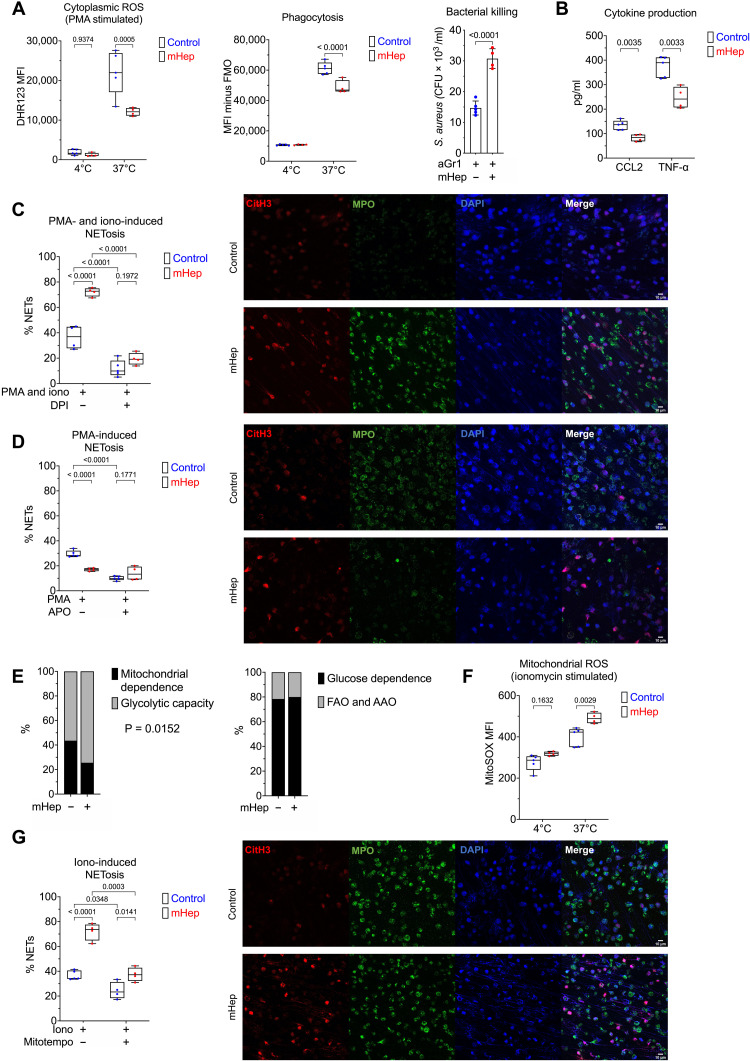
Fig. 3. Functional alteration of neutrophils in serum iron deficiency.
(A) Phenotyping ex vivo of isolated BM neutrophils (obtained from mice treated as in Fig. 2A). Dihydrorhodamine 123 (DHR123) fluorescence as a reporter for cytoplasmic ROS in PMA-stimulated cells. Phagocytosis of GFP-labeled E. coli. FMO, fluorescence minus one. Two-way ANOVA. Mean, quartiles, and range. Bacterial killing of S. aureus, resolved as colony-forming units of S. aureus recovered after coculture with neutrophils. Unpaired t test. Means ± SD. (B) Supernatant CCL2 and TNF levels produced by zymosan-stimulated neutrophils measured by enzyme-linked immunosorbent assay (ELISA). Unpaired t test. Mean, quartiles, and range. (C) NETosis was evaluated in isolated BM neutrophils after stimulation ex vivo with PMA and ionomycin ± DPI. A minimum of 250 cells from two replicates per sample counted. Two-way ANOVA. Mean, quartiles, and range. Representative microscopy images taken at ×20 magnification. (D) NETosis was evaluated in isolated BM neutrophils after stimulation ex vivo with PMA ± apocynin (APO). A minimum of 250 cells from two replicates per sample counted. Two-way ANOVA. Mean, quartiles, and range. Representative microscopy images taken at ×20 magnification. (E) SCENITH (single-cell metabolism by profiling translation inhibition) analysis of Ly6G+ BM neutrophils from experiment described in Fig. 1A, analyzing proportional shifts in O-propargyl-puromycin (OPP) incorporation after ex vivo treatment with metabolic inhibitors as detailed in Materials and Methods. Two-way ANOVA with matching for sample. Reported P value is the effect of mHep treatment. Mean. FAO, fatty acid oxidation; AAO,amino acid oxidation. (F) MitoSOX fluorescence as a reporter for mitoROS in ionomycin stimulated isolated BM neutrophils. Two-way ANOVA. Mean, quartiles, and range. (G) NETosis was evaluated in isolated BM neutrophils after stimulation ex vivo with ionomycin (iono) ± mitoTEMPO. A minimum of 250 cells from two replicates per sample counted. Two-way ANOVA. Mean, quartiles, and range. Representative microscopy images taken at ×20 magnification.