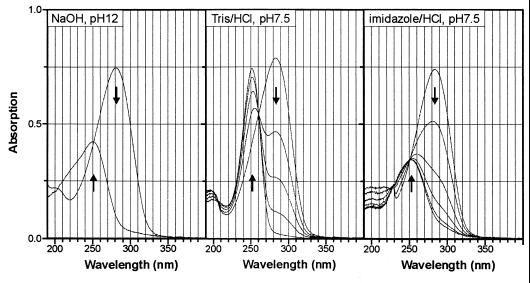
FIG. 5.
Formation of 2-chloromaleylacetate and compound X (a reaction product with Tris) during nonenzymatic turnover of 2-chloro-cis-dienelactone in aqueous NaOH and in the presence of different buffers, respectively. For alkaline hydrolysis, the reaction mixture (1 ml) contained 0.05 mM 2-chloro-cis-dienelactone in water. After recording the spectra using water as the reference, we added 2 μl of 2 M NaOH (4 μmol) to both cuvettes and recorded an overlay spectrum after 0.5 min. For the turnover in buffer, reaction mixtures (1 ml) contained 100 mM buffer and 0.05 mM 2-chloro-cis-dienelactone. Reference cuvettes contained the same buffer. Five spectra were recorded after 0.5, 8, 17, 30, and 60 min (Tris-HCl, pH 7.5) and after 0.5, 60, 120, 180, 240, and 270 min (imidazole-HCl, pH 7.5).