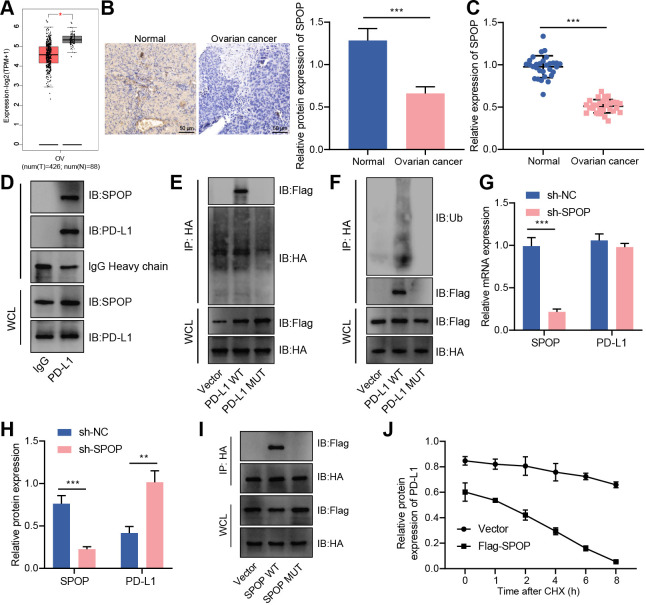
Figure 4.
CUL3 promoted PD-L1 ubiquitination and degradation by forming a complex with SPOP in ovarian cancer cells. (A) Analysis of the expression of SPOP in ovarian cancer (normal group, n=38; cancer group, n=426) by gene expression profiling interactive analysis database. (B) Immunohistochemistry of SPOP protein expression in ovarian cancer tissue. (C) Detection of the mRNA expression of SPOP in adjacent normal tissues and ovarian cancer tissues (n=30) by RT-qPCR. (D) Detection of the interaction between PD-L1 and SPOP in SKOV3 cells by Co-IP experiment. (E) Co-IP experiment detected the interaction relationship between PD-L1 and SPOP in each group of SKOV3 cells. (F) Co-IP experiment detected the interaction relationship between SPOP and PD-L1. (G) RT-qPCR detection of the mRNA levels of SPOP and PD-L1 in SKOV3 cells after knocking down SPOP. (H) Western blot detection of the protein levels of SPOP and PD-L1 in SKOV3 cells after knocking down SPOP. (I) Co-IP experiment detected the interaction between PD-L1 and SPOP in SKOV3 cells after mutation of the binding site of SPOP and PD-L1. (J) Western blot detection of the protein degradation rate of PD-L1 in SKOV3 cells transfected with Flag-SPOP. **p<0.01, ***p<0.001. All experiment was repeated three times. Co-IP, co-immunoprecipitation; CUL3, cullin 3; mRNA, messenger RNA, PD-L1, programmed death ligand-1; RT-qPCR, reverse transcription quantitative PCR; SPOP, speckle type POZ protein.