Abstract
Coronavirus disease 2019 (COVID-19) is a systemic illness with an increased host inflammatory response that affects multiple extra-pulmonary organs, including the gastrointestinal tract. Abnormalities in liver biochemistry have been observed in a significant proportion of patients with COVID-19 upon admission, and this proportion increases with hospitalization. These abnormalities are typically manifested as elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels, with less frequently detected elevations in the levels of cholestatic enzymes. Elevated aminotransaminase levels have been linked to an increased risk of mortality and complications, indicating the severity of COVID-19 infection. The present study evaluated the prevalence and the baseline factors associated with the development of acute hepatitis (ΑΗ), liver injury (LI) and associated patterns, as well as the presence of abnormalities in the levels of aminotransferases at discharge in the same cohort. For this purpose, 1,304 patients with confirmed COVID-19 infection were enrolled in the study. According to the results obtained, AST levels at baseline were the only independent factor for AH during hospital stay, while AST, alkaline phosphatase and ferritin levels were independent baseline factors for the development of LI. The patients with hepatocellular, compared to those with cholestatic LI, exhibited similar survival rates, as well as similarities in the development of acute kidney injury and the need for oxygen via high-flow nasal cannula and/or mechanical ventilation. In addition, age and ALT were independent risk factors for persistent abnormal values of AST and ALT at discharge.
Keywords: coronavirus disease 2019, SARS-CoV-2, acute liver injury, acute hepatitis, prognosis
Introduction
Coronavirus disease 2019 (COVID-19) is considered a systematic disease with an enhanced host inflammatory response (1) affecting several extra-pulmonary organs, such as the gastrointestinal system (2,3). Abnormalities in liver biochemistry have been recorded in over half of the patients with COVID-19 upon admission and this proportion is increased during hospitalization (4,5). These abnormalities are usually presented as elevations in the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT), while less frequently, increased levels of cholestatic enzymes [i.e., alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT)] and bilirubin have been observed (4). The development of acute hepatitis (AH) is defined as AST and/or ALT levels >400 IU/l, and the presence of liver injury (LI) is defined as an elevation in ALT levels [(>3-fold the upper limit of normal (ULN)] or at least as a moderate elevation in the levels of ALP or total bilirubin (>2-fold the ULN) (6). Although the pathogenetic mechanisms associated with COVID-19-induced LI (usually defined as a combination of aminotransferases/cholestatic enzymes/bilirubin abnormalities) (7) have not yet been elucidated, elevated levels of aminotransaminases have been shown to be associated with a higher risk of mortality (6) and complications, indicating the severity of COVID-19 and the intensity of hyperinflammation syndrome and cytokine storm observed during severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection (5,8). Of note, although the majority of patients develop a hepatocellular pattern of LI (i.e., predominance of AST/ALT elevation, compared to ALP/γ-GT), in some patients, a cholestatic pattern of LI has been recorded (i.e., an elevation in the levels of cholestatic enzymes and bilirubin) without a clear clinical impact of this finding (9).
It should be mentioned that abnormalities in the levels of aminotransferase are usually mild during COVID-19 infection; however, in some patients, these can progress to AH. Although the development of AH has been found to be associated with a worse prognosis (9), the baseline risk factors related to AH during hospitalization have not yet been fully clarified, since large cohorts need to be assessed to evaluate this rare biochemical phenomenon. Fortunately, only a few cases of patients progressing to acute liver failure (ALF) have been recorded in the literature (10), while in the majority of patients with abnormalities in biochemical liver test results, values gradually return to normal levels (11). In a previous study, the authors evaluated the prevalence, clinical impact and the risk factors associated with the development of LI (5). Using the same cohort of patients with COVID-19, the present study aimed to evaluate, for the first time (to the best of our knowledge), the prevalence, clinical impact and the risk factors associated with the development of AH and LI, as well as to assess the association of the LI pattern with the course of COVID-19 infection. In addition, the present study aimed to estimate the prevalence of patients discharged with abnormal aminotransferase levels in a single-center study on Greek patients hospitalized due to COVID-19.
Patients and methods
Patient population
Consecutive adult patients who had been hospitalized with documented COVID-19 infection in the non-ICU ward at Laiko General Hospital, Athens, Greece, between March, 2020 and October, 2021, were evaluated retrospectively. The inclusion criteria were an age ≥18 years at the time of hospitalization and at least one positive reverse transcription-polymerase chain reaction (RT-PCR) test result for SARS-CoV-2 performed on a nasopharyngeal swab specimen. The exclusion criteria were pregnancy, unavailability of detailed medical records, or a hospitalization period of <3 days. All patients were followed-up until discharge or mortality. The study protocol was approved by the Data Protection Officer and Institutional Review Board of Laiko General Hospital (protocol no. 770/17-12-2021) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki (as revised in 2000). All patients provide written informed consent.
Baseline evaluation
Clinical variables upon admission were recorded, including age, sex, body mass index (BMI) and the consumption of alcohol, as well as past medical history, including the use of anti-hypertensive and anti-diabetic drugs, while severe (or class II) obesity was defined as a BMI >35 kg/m2 (10). Laboratory variables during the first 24 h of admission were obtained from the electronic medical record system, including white blood cell count (WBC) with differential, platelets (PLTs), protein, albumin, creatinine, total bilirubin, clotting profile [international normalized ratio (INR), fibrinogen and D-dimers], aminotransferases (AST and ALT), ALP, γ-GT, lactate dehydrogenase (LDH), C-reactive protein (CRP) and ferritin. In addition, the HBsAg/anti-HCV serological status was recorded whenever available. Elevated serum levels of aminotransferases at baseline were defined as ALT or AST levels >40 IU/l, while ULN ranges of total bilirubin, ALP and γ-GT were 1.2 mg/dl, 104 and 55 IU/l, respectively.
During their hospitalization, all included patients received supportive care with a prophylactic dose of low-molecular-weight heparin (or therapeutic dose in cases of confirmed thromboembolic events), fluid and electrolyte replacement therapy, and oxygen supplementation (delivered by nasal catheters, Venturi masks, or high-flow nasal cannula) as required, according to the national guidelines [https://www.hts.org.gr/assets/anatheorimenos%20algorithmos%20nosileyomenon_Feb2022.pdf]. The administration of all medications was at the discretion of the attending physician. For COVID-19-associated pneumonia, the standard of care included the administration of remdesivir and dexamethasone. The administration of baricitinib and tocilizumab was decided on a case-by-case basis. In addition, during their hospital stay, the maximum values of AST, ALT, ALP, γ-GT and bilirubin were recorded. The development of AH was defined as AST and/or ALT levels >400 IU/l; the presence of LI during hospital stay was defined as an elevation of ALT levels (>3-fold ULN) or at least a moderate elevation in ALP or total bilirubin levels (>2-fold ULN) during hospital stay (6). The definitions for AH and LI were universal for all patients, irrespectively of the presence of underlying liver disease. Furthermore, in those patients with LI, the pattern of LI was further characterized as hepatocellular (R ≥5), cholestatic (R ≤2), or mixed (R between 2 and 5) [R was calculated according to the formula R=(ALT value/ALT ULN)/(ALP value/ALP ULN] (12). Finally, in the subgroup of patients with available discharge laboratory data, the serum levels of aminotransferases (AST and ALT) were recorded. The primary outcome of the study was to identify the baseline factors associated with the development of AH and LI during hospitalization, as well as the presence of abnormal aminotransferase levels at discharge. Secondary outcomes included the evaluation of the impact of AH and LI on in-hospital mortality, the need for high-flow nasal cannula and/or intubation, as well as the development of acute kidney injury (AKI) during hospitalization. The latter was defined as an increase in serum creatinine levels 2-fold the baseline values.
Statistical analysis
Continuous variables in the present study cohort are presented as the mean ± standard deviation (normally distributed) or median with range (non-normally distributed), while categorical variables are expressed as frequencies or percentages. Comparisons of variables between patients were performed using Mann-Whitney U tests for continuous variables, respectively, and the Chi-squared test for categorical variables. In univariate analysis, the baseline variables were included, while multivariate Cox regression analysis was used to identify the baseline variables independently associated with the development of AH and LI in the total cohort. The discriminative ability of the independent baseline factors was evaluated by using the area under the receiver operating characteristic curve (ROC) (13). The patient's outcomes were calculated using Kaplan-Meier analysis and compared with the log rank sum test. A P-value <0.05 (two-tailed) was considered to indicate a statistically significant difference. Statistical analysis was conducted using SPSS softwre (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0., IBM Corp.) and MedCalc for Windows (MedCalc Software, version 20.114).
Results
Baseline characteristics
A total of 1,304 patients with COVID-19 infection (757 males, age 64±17 years) were evaluated. All patients had clinical manifestations of COVID-19 infection, including fever and respiratory symptoms. However, radiological findings of lower respiratory tract infection were not present in all patients. The number of patients who received antibiotics was too small to perform a statistical analysis. Furthermore, the doses of remdesivir and dexamethasone were the same for all patients. In addition, the majority of the patients received a single dose of tocilizumab (dose of 400 mg). In total, 37 (2.8%) patients were ΗΒsAg-positive, while 11 (0.8%) were anti-HCV-positive. Upon admission, 864 (66.2%) and 975 (74.8%) patients had normal AST and ALT values (i.e., ≤40 IU/l), respectively, while only six (0.4%) patients had AST or ALT levels >400 IU/l. Patients with viral hepatitis compared to those without viral hepatitis had similar baseline (at admission) aminotransferase levels. In patients with viral hepatitis, the baseline value of AST was 55±17 IU/l and that of ALT was 47±15 IU/l. In addition, 49 (3.7%), 153 (11.7%) and 302 (23.1%) of the patients had abnormal levels of total bilirubin (i.e., >1.2 mg/dl), ALP (i.e., >104 IU/l) and γ-GT (i.e., >55 IU/l), respectively. The baseline clinical and laboratory characteristics of the patients are presented in Table I.
Table I.
Baseline clinical and laboratory characteristics of the 1,304 patients with COVID-19 infection.
| Variable | Patients (n=1,304) |
|---|---|
| Age (mean ± SD, years) | 64±17 |
| Sex, n (%) | |
| Male | 757(58) |
| Female | 547(42) |
| Co-morbidities, n (%) | |
| Diabetes mellitus | 245 (18.7) |
| Severe (class II) obesity (BMI ≥35 kg/m²) | 79(6) |
| Arterial hypertension | 422(32) |
| Gastrointestinal symptoms | 144(11) |
| AST (median, range, IU/l) | 33 (4-957) |
| ALT (median, range, IU/l) | 25 (3-993) |
| ALP (median, range, IU/l) | 65 (12-1335) |
| γ-GT (median, range, IU/l) | 35 (6-1304) |
| Total bilirubin (median, range, mg/dl) | 0.46 (0.11-33) |
| LDH (median, range, IU/l) | 324 (9-3552) |
| Albumin (median, range, g/dl) | 3.9 (1.8-5.4) |
| CRP (median, range, mg/l) | 55 (0.7-478) |
| INR (median, range) | 1.0 (0.7-11.3) |
| D-Dimers (median, range, mg/dl) | 0.9 (0.09-52) |
| Fibrinogen (median, range, mg/dl) | 536 (40-1074) |
| Ferritin (median, range, ng/ml) | 535 (10-2940) |
| WBC (median, range, x109/l) | 6.1 (1.2-45) |
| PLTs (mean ± SD, x109/l) | 211±94 |
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; INR, international normalized ratio; CRP, C-reactive protein; WBC, white blood count; PLTs, platelets; BMI: body mass index.
Baseline variables associated with the development of AH
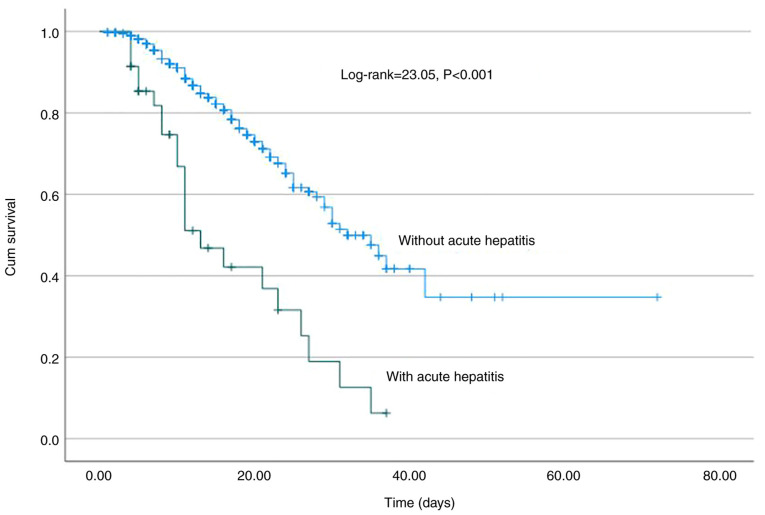
In total, 35 patients (2.7%) developed AH during hospitalization after 5 (range, 3-40) days. In addition, 4 patients with viral hepatitis developed AH. However, none of the patients developed acute liver failure with an increase in the INR or encephalopathy. In the univariate analysis, AST (HR, 1.008; 95% CI, 1.006-1.009; P<0.001), ALT (HR, 1.007; 95% CI, 1.006-1.009; P<0.001), LDH (HR, 1.002; 95% CI, 1.001-1.003; P<0.001), ferritin (HR, 1.003; 95% CI, 1.001-1.004; P=0.007), the administration of remdesivir (HR, 0.41; 95% CI, 0.19-0.89; P=0.025), and HBsAg or anti-HCV positivity (HR, 4.05; 95% CI, 1.18-13.9; P=0.026) were the baseline factors associated with the development of AH (Table II). In the multivariate analysis, AST at baseline (HR, 1.008, 95% CI, 1.006-1.011; P<0.001) was the only independent factor for AH during hospital stay with efficient discriminative ability [area under the curve (AUC), 0.72; 95% CI, 0.65-0.81, P<0.05) (Fig. 1). Of note, the development of AH was associated with a higher risk of mortality (log-rank, 23.05, P<0.001) (Fig. 2) and AKI (log-rank, 33.5, P<0.001) (data not shown); however, it was not associated with the need for oxygen via high-flow nasal cannula and/or intubation and mechanical ventilation (log-rank, 0.92, P=0.34) (data not shown).
Table II.
Baseline risk factors associated with acute hepatitis during hospital stay in 1,304 patients with COVID-19 infection (univariate and multivariate analysis).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Hazard ratio | P-value | 95% Confidence interval Lower limit | 95% Confidence interval Upper limit | Hazard ratio | P-value | 95% Confidence interval Lower limit | 95% Confidence interval Upper limit |
| Age, years | 0.99 | 0.61 | 0.97 | 1.054 | ||||
| Sex, male | 0.89 | 0.75 | 0.45 | 1.72 | ||||
| Co-morbidities | ||||||||
| Diabetes mellitus | 1.36 | 0.44 | 0.62 | 2.99 | ||||
| Severe (class II) obesity (BMI ≥35 kg/m²) | 1.42 | 0.56 | 0.43 | 4.71 | ||||
| Arterial hypertension | 1.31 | 0.47 | 0.63 | 2.68 | ||||
| Gastrointestinal symptoms | 1.97 | 0.17 | 0.74 | 5.33 | ||||
| AST (IU/l) | 1.008 | <0.001 | 1.006 | 1.009 | 1.008 | <0.001 | 1.006 | 1.011 |
| ALT (IU/l) | 1.007 | <0.001 | 1.006 | 1.009 | ||||
| ALP (IU/l) | 1.002 | 0.51 | 0.99 | 1.006 | ||||
| γ-GT (IU/l) | 1.002 | 0.19 | 0.99 | 1.005 | ||||
| Total bilirubin (mg/dl) | 0.99 | 0.34 | 0.908 | 1.095 | ||||
| LDH (IU/l) | 1.002 | <0.001 | 1.001 | 1.003 | ||||
| Albumin (g/dl) | 0.99 | 0.64 | 0.97 | 1.017 | ||||
| CRP (mg/l) | 1.00 | 0.81 | 0.99 | 1.001 | ||||
| INR | 0.98 | 0.85 | 0.85 | 1.10 | ||||
| D-Dimers (mg/dl) | 1.00 | 0.99 | 0.99 | 1.001 | ||||
| Fibrinogen (mg/dl) | 1.001 | 0.27 | 0.99 | 1.003 | ||||
| Ferritin (ng/ml) | 1.003 | 0.007 | 1.001 | 1.004 | ||||
| WBC (x109/l) | 0.99 | 0.85 | 0.93 | 1.06 | ||||
| PLTs (x109/l) | 0.99 | 0.26 | 0.99 | 1.002 | ||||
| HBsAg (+) or anti-HCV (+) | 4.05 | 0.026 | 1.18 | 13.9 | ||||
| COVID-19 medication | ||||||||
| Remdesivir | 0.41 | 0.025 | 0.19 | 0.89 | ||||
| Dexamethasone | 1.21 | 0.76 | 0.36 | 3.98 | ||||
| Tocilizumab | 1.98 | 0.09 | 0.88 | 4.47 | ||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; INR, international normalized ratio; CRP, C-reactive protein; WBC, white blood count; PLTs, platelets; BMI: body mass index.
Figure 1.
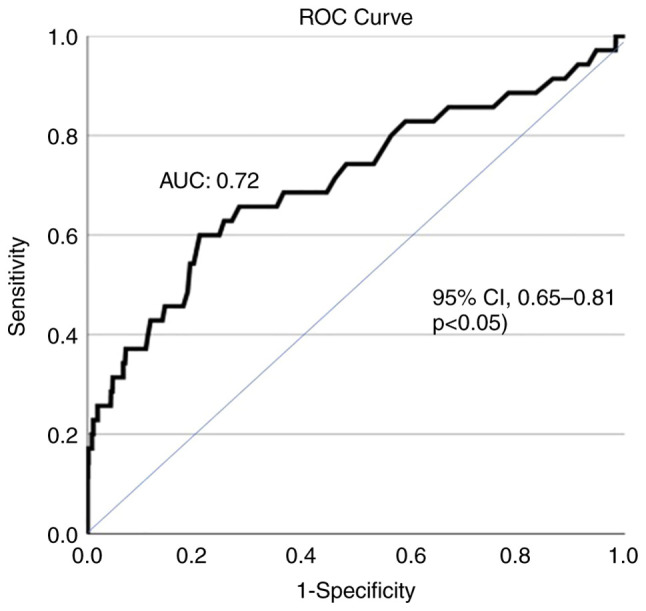
Area under the receiver operating characteristic curves (AUC) illustrating that AST levels at baseline had a notable discriminative ability (AUC, 0.72; 95% CI, 0.65-0.81, P<0.05) for the development of acute hepatitis during hospital stay in 1,304 patients with COVID-19 infection. The optimal cut-off value for AST was 50.5 IU/l (sensitivity, 60%; specificity, 79%). AST, aspartate aminotransferase; AUC, area under the curve.
Figure 2.
Kaplan-Meier survival curve. Significantly worse survival was observed in patients with acute hepatitis (log-rank, 23.05, P<0.001).
Baseline variables associated with the development of LI
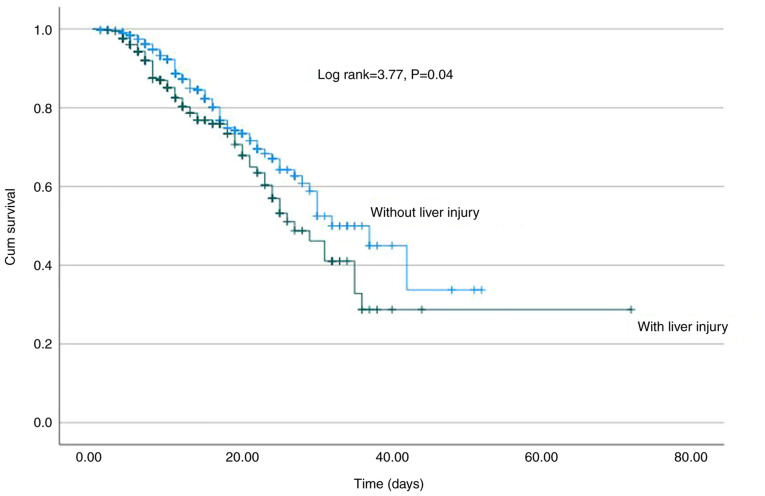
A total of 336 (25.8%) developed LI during hospitalization after 3 (1-36) days. In the univariate analysis, AST (HR, 1.005; 95% CI, 1.004-1.006; P<0.001), ALT (HR, 1.005; 95% CI, 1.004-1.005; P<0.001), ALP (HR, 1.003; 95% CI, 1.002-1.004; P<0.001), γ-GT (HR, 1.003; 95% CI, 1.002-1.003; P<0.001), LDH (HR, 1.002; 95% CI, 1.001-1.003; P<0.001), ferritin (HR, 1.002; 95% CI, 1.001-1.003; P<0.001) and D-dimers (HR, 1.001; 95% CI, 1.00-1.002; P=0.015) were the baseline factors associated with the development of LI (Table III). In the multivariate analysis, AST (HR, 1.004; 95% CI, 1.002-1.003; P<0.001), ALP (HR, 1.003; 95% CI, 1.002-1.004; P<0.001) and ferritin (HR, 1.001; 95% CI, 1.00-1.002; P=0.005) were the independent baseline factors for LI development, while ALP exhibited the optimal discriminative ability (AUC, 0.73; 95% CI, 0.68-0.78) followed by AST (AUC, 0.63; 95% CI, 0.59-0.67) and ferritin (AUC, 0.61; 95% CI, 0.57-0.65) (Fig. 3). Of note, LI development was associated with a higher risk for mortality (log-rank, 3.77; P=0.04) (Fig. 4), but neither with AKI (log-rank, 0.83; P=0.36) nor the need for oxygen via high-flow nasal cannula and/or mechanical ventilation (log-rank 1.31; P=0.25) (Table III).
Table III.
Baseline risk factors associated with liver injury (LI) during hospital stay in 1.304 patients with COVID-19 infection (univariate and multivariate analysis).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Hazard ratio | P-value | 95% Confidence interval Lower limit | 95% Confidence interval Upper limit | Hazard ratio | P-value | 95% Confidence interval Lower limit | 95% Confidence interval Upper limit |
| Age, years | 0.992 | 0.013 | 0.985 | 0.998 | ||||
| Sex, male | 0.83 | 0.12 | 0.669 | 1.049 | ||||
| Co-morbidities | ||||||||
| Diabetes mellitus | 0.91 | 0.49 | 0.67 | 1.21 | ||||
| Severe (class II) obesity (BMI ≥35) | 0.82 | 0.38 | 0.53 | 1.27 | ||||
| Arterial hypertension | 0.87 | 0.24 | 0.68 | 1.11 | ||||
| Gastrointestinal symptoms | 1.13 | 0.46 | 0.81 | 1.59 | ||||
| AST (IU/l) | 1.005 | <0.001 | 1.004 | 1.006 | 1.002 | <0.001 | 1.001 | 1.003 |
| ALT (IU/l) | 1.005 | <0.001 | 1.004 | 1.006 | ||||
| ALP (IU/l) | 1.003 | <0.001 | 1.002 | 1.004 | 1.003 | <0.001 | 1.002 | 1.004 |
| γ-GT (IU/l) | 1.003 | <0.001 | 1.002 | 1.004 | ||||
| Total bilirubin (mg/dl) | 0.979 | 0.93 | 0.88 | 1.016 | ||||
| LDH (IU/l) | 1.001 | <0.001 | 1.00 | 1.002 | ||||
| Albumin (g/dl) | 0.99 | 0.63 | 0.99 | 1.003 | ||||
| CRP (mg/l) | 1.0 | 0.98 | 0.99 | 1.002 | ||||
| INR | 0.99 | 0.86 | 0.97 | 1.018 | ||||
| D-Dimers (mg/dl) | 1.001 | 0.015 | 1.0 | 1.002 | ||||
| Fibrinogen (mg/dl) | 1.001 | 0.33 | 0.99 | 1.002 | ||||
| Ferritin (ng/ml) | 1.01 | <0.001 | 1.001 | 1.02 | 1.001 | 0.005 | 1.00 | 1.002 |
| WBC (x109/l) | 0.995 | 0.62 | 0.99 | 1.001 | ||||
| PLTs (x109/l) | 1.0 | 0.79 | 0.99 | 1.001 | ||||
| HBsAg (+) or anti-HCV (+) | 1.42 | 0.21 | 0.83 | 2.43 | ||||
| COVID-19 medication | ||||||||
| Remdesivir | 0.79 | 0.13 | 0.59 | 1.068 | ||||
| Dexamethasone | 1.05 | 0.79 | 0.72 | 1.51 | ||||
| Tocilizumab | 0.91 | 0.56 | 0.67 | 1.24 | ||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; INR, international normalized ratio; CRP, C-reactive protein; WBC, white blood count; PLTs, platelets.
Figure 3.
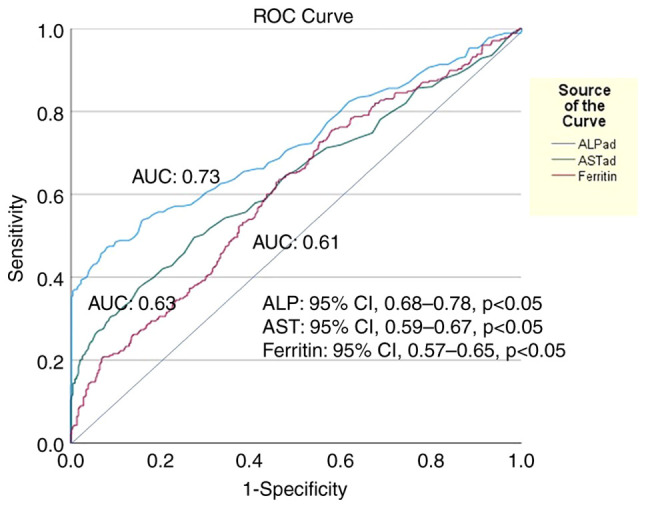
Area under the receiver operating characteristic curves (AUC) illustrating that (ALP) at baseline had the best discriminative ability (AUC, 0.73; 95% CI, 0.68-0.78, P<0.05) followed by AST (AUC, 0.63; 95% CI, 0.59-0.67, P<0.05) and ferritin (AUC, 0.61; 95% CI, 0.57-0.65, P<0.05) for the development of acute liver injury during hospital stay in 1,304 patients with COVID-19 infection. The optimal cut-off value for ALP was 84.5 IU/l (sensitivity, 55%; specificity, 82%). ALP, alkaline phosphatase; AST, aspartate aminotransferase; AUC, area under the curve.
Figure 4.
Kaplan-Meier survival curve. Significantly worse survival was observed in patients with liver injury (log-rank, 3.77, P=0.04).
Baseline variables associated with the development of hepatocellular or cholestatic LI
Among the patients with LI (n=336), 129 patients (38.4%) developed hepatocellular LI (R factor ≥5), 159 patients (47.3%) cholestatic LI (R factor ≤2) and 48 patients (14.3%) developed mixed LI (R factor 2-5). The patients with hepatocellular, compared to those with cholestatic LI, exhibited similar survival rates (log-rank, 0.37; P=0.54), as well as similarities in AKI development (log-rank, 0.1; P=0.77) and the need for oxygen via high-flow nasal cannula and/or mechanical ventilation (log-rank, 1; P=0.99) (data not shown) The viral hepatitis status was not significantly associated with LI.
Baseline variables associated with persistent abnormal aminotransferases at discharge
In the subgroup of patients who were alive with available data at discharge (n=686), 372 patients had AST and ALT levels within normal ranges, while 314 patients had abnormal values of AST and/or ALT after 8 (range, 3-40) days of hospitalization. In the univariate analysis, age (HR, 0.98; 95% CI, 0.97-0.99; P<0.001), sex (HR, 0.63; 95% CI, 0.51-0.79; P<0.001), AST (HR, 1.006; 95% CI, 1.004-1.008; P<0.001), ALT (HR, 1.004; 95% CI, 1.002-1.005; P<0.001), total bilirubin (HR, 1.006; 95% CI, 1.0-1.012; P=0.04) and ferritin (HR, 1.002; 95% CI, 1.001-1.003; P=0.003) were the baseline factors associated with the presence of abnormal AST/ALT at discharge (Table IV). In the multivariate analysis, age (HR, 0.98; 95% CI, 0.97-0.99; P<0.001) and ALT (HR, 1.005; 95% CI, 1.003-1.007; P<0.001) were the independent risk factors. In addition, baseline ALT levels had a notable discriminative ability (AUC, 0.78; 95% CI, 0.74-0.82), better than the discriminative ability of age (AUC, 0.53; 95% CI, 0.48-0.58; P=0.02) for the presence of abnormal AST/ALT at discharge (data not shown).
Table IV.
Baseline risk factors of 686 patients for the presence of normal ALT/AST at discharge after hospitalization for COVID-19 infection (univariate and multivariate analysis).
| Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Hazard ratio | P-value | 95% Confidence interval Lower limit | 95% Confidence interval Upper limit | Hazard ratio | P-value | 95% Confidence interval Lower limit | 95% Confidence interval Upper limit |
| Age, years | 0.98 | <0.001 | 0.97 | 0.99 | 0.98 | <0.001 | 0.97 | 0.99 |
| Sex, male | 0.63 | <0.001 | 0.51 | 0.79 | ||||
| Co-morbidities | ||||||||
| Diabetes mellitus | 0.61 | 0.001 | 0.45 | 0.82 | ||||
| Severe (class II) obesity (BMI ≥35) | 1.15 | 0.45 | 0.79 | 1.69 | ||||
| Arterial hypertension | 0.82 | 0.71 | 0.65 | 1.02 | ||||
| Gastrointestinal symptoms | 1.26 | 0.14 | 0.92 | 1.73 | ||||
| AST (IU/l) | 1.006 | <0.001 | 1.004 | 1.008 | ||||
| ALT (IU/l) | 1.004 | <0.001 | 1.002 | 1.005 | 1.005 | <0.001 | 1.003 | 1.007 |
| ALP (IU/l) | 1.00 | 0.66 | 0.99 | 1.002 | ||||
| γ-GT (IU/l) | 1.001 | 0.12 | 0.99 | 1.002 | ||||
| Total bilirubin (mg/dl) | 1.006 | 0.048 | 1.00 | 1.012 | ||||
| LDH (IU/l) | 1.001 | 0.27 | 0.98 | 1.002 | ||||
| Albumin (g/dl) | 1.002 | 0.16 | 0.99 | 1.004 | ||||
| CRP (mg/l) | 1.00 | 0.85 | 0.99 | 1.001 | ||||
| INR | 0.93 | 0.54 | 0.75 | 1.16 | ||||
| D-Dimers (mg/dl) | 0.97 | 0.09 | 0.94 | 1.002 | ||||
| Fibrinogen (mg/dl) | 1.00 | 0.98 | 0.99 | 1.001 | ||||
| Ferritin (ng/ml) | 1.002 | 0.003 | 1.001 | 1.003 | ||||
| WBC (x109/l) | 1.00 | 0.13 | 0.99 | 1.001 | ||||
| PLTs (x109/l) | 1.00 | 0.68 | 0.99 | 1.001 | ||||
| HBsAg (+) or anti-HCV (+) | 0.88 | 0.76 | 0.39 | 2.01 | ||||
| COVID-19 medication | ||||||||
| Remdesivir | 0.93 | 0.59 | 0.71 | 1.22 | ||||
| Dexamethasone | 0.93 | 0.69 | 0.67 | 1.29 | ||||
| Tocilizumab | 0.69 | 0.07 | 0.48 | 1.01 | ||||
AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; LDH, lactate dehydrogenase; INR, international normalized ratio; CRP, C-reactive protein; WBC, white blood count; PLTs, platelets.
Discussion
In the present single-center study including a large cohort of patients with confirmed COVID-19 infection, the prevalence and the baseline factors associated with the development of AH (defined as AST and/or ALT >400 IU/l) were evaluated. In addition, the impact of LI development (defined as an elevation of ALT levels >3-fold the ULN or ALP or total bilirubin >2-fold the ULN) and its pattern (hepatocellular or cholestatic) on survival during hospital stay were assessed. Previous studies have demonstrated that abnormalities in aminotransferase levels, derived through direct SARS-CoV-2 or drug-induced liver injury, ischemic damage, or non-hepatic mechanisms, are frequently observed during COVID-19 infection and they have been shown to be associated with the need for intubation and a higher mortality rate (4,5). In the large cohort in the present study, literature data were confirmed, since a relatively high prevalence of AST/ALT abnormalities were observed upon admission, while a lower proportion of patients with ALP/γ-GT or bilirubin above the ULN were recorded.
Although these cases very rarely progress to ALF, it was recently reported that out 624 patients infected with COVID-19, 43 (6.9%) developed ALF during their hospitalization (14). However, this proportion appears to be very high. To the best of our knowledge, no other study to date has yet confirmed this finding, and it is outside of the common daily clinical experience. In fact, in the literature, only a few cases of ALF upon admission or during hospital stay in patients with COVID-19 have been reported (10,15). In the present study, 35 (2.7%) patients developed AH during hospital stay after 5 (1-40) days, while none of the patients progressed to ALF failure with INR prolongation or encephalopathy. In the literature, several studies have recorded AST/ALT abnormalities of 3- to 5-fold higher than ULN during hospitalization (6,16,17), while only a few studies have evaluated the prevalence of AH (i.e., AST or ALT >10-fold the ULN) development during COVID-19 hospitalization (5,18). In a recent study (7), AH was recorded in 87 (5.6%) of 1,555 patients with COVID-19, while in another study (19), none of the 554 patients developed aminotransferase levels >15-fold the ULN, while no data were provided for patients with AST/ALT levels >10-fold the ULN. In two studies (5,18), similar to the present study, none of the patients developed ALF. Nevertheless, the prognostic impact of severe AST/ALT abnormalities was confirmed in the present study cohort, since AH was associated with higher risk of mortality (log-rank 23.05; P<0.001) and AKI (log-rank, 33.5; P<0.001). To the best of our knowledge, the present study is the first in which the baseline factors associated with AH development were evaluated: In multivariate analysis, AST at baseline (HR, 1.008; 95% CI, 1.006-1.011; P<0.001) was the only independent factor for AH during hospital stay with a notable discriminative ability (AUC, 0.72; 95% CI, 0.65-0.81) (Fig. 1).
In the present study cohort, it was found that 25.8% of patients developed LI after 3 (range 1-36) days of hospitalization. In addition, AST (HR, 1.002; 95% CI, 1.001-1.003; P<0.001), ALP (HR, 1.003; 95% CI, 1.002-1.004; P<0.001) and ferritin (HR, 1.001, 95% CI, 1.00-1.002; P=0.005) were the independent baseline factors for LI development, while similar to a previous study, LI was associated with a higher risk of mortality (log-rank, 3.77; P=0.04) (5). Although in a previous study (19) the LI pattern was associated with mortality, this was not confirmed in the present study cohort, since the patients with hepatocellular, compared to those with cholestatic LI, had similar survival rates (log-rank 0.37, P=0.54), as well as AKI development and the need for high-flow oxygen/mechanical ventilation. Although the patients with hepatocellular, compared to those with cholestatic LI, had significantly higher baseline inflammatory markers (CRP and ferritin), possibly indicating a more intense inflammatory response, this difference was not associated with a worse outcome (data not shown).
Finally, based on the available data and similar to a previous study (10), 46% (314/686) of the patients were discharged with abnormal AST and/or ALT after 8 (1-40) days of hospitalization. Interestingly, age (HR, 0.98; 95% CI, 0.97-0.99; P<0.001) and ALT (HR, 1.005; 95% CI, 1.003-1.007, P<0.001) were the independent risk factors, while ALT had a notable discriminative ability (AUC, 0.78; 95% CI, 0.74-0.82) (data not shown).
The present study had several limitations, including the fact that it was a single-center retrospective study. However, all eligible patients were included based on the electronic medical record system of the hospital. Another limitation of the study was that patients in the intensive care unit with COVID-19 were not included. The incidence of liver injury is higher in patients with more severe COVID-19 than in patients with mild disease. Finally, an additional limitation of the study is that the association between hypoxia or shock, that are most frequently observed in critical COVID-19, and the development of LI and AH was not evaluated. However, the present study evaluated the association between AH and LI with the need for mechanical ventilation and AKI.
In conclusion, to the best of our knowledge, the present study is the first in which the prevalence and the baseline factors associated with the development of AH, LI and its pattern, as well as the presence of aminotransferase abnormalities at discharge were evaluated in the same cohort. AST levels at baseline were the only independent factor for AH during hospital stay, while AST, ALP and ferritin levels were the independent baseline factors for LI development. Patients with hepatocellular, compared to those with cholestatic LI, had similar survival rates, similarities in AKI development and the need for oxygen via high-flow nasal cannula and/or mechanical ventilation. Apart from age, and ALT was an independent risk factor for the presence of abnormal AST/ALT at discharge.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
EC, MS and NVS conceptualized the study. TB, VEG, SM, MT, SS, PMV, DB, IE, PP, GK, MAd, SA, OC, EA, AA, MAt and KT advised on patient care and medical treatment, made substantial contributions to conception and design and acquisition of data, and wrote and prepared the draft of the manuscript. EC, MS, DAS and NVS analyzed the data and provided critical revisions. EC and NVS confirm the authenticity of all the data. All authors contributed to manuscript revision, and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study protocol was approved by the Data Protection Officer and Institutional Review Board of Laiko General Hospital (Athens, Greece) and conformed to the ethical guidelines of the 1975 Declaration of Helsinki (as revised in 2000).
Patient consent for publication
Not applicable.
Competing interests
DAS is the Editor-in-Chief for the journal, but had no personal involvement in the reviewing process, or any influence in terms of adjudicating on the final decision, for this article. The other authors declare that they have no competing interests.
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhurwal A, Minacapelli CD, Orosz E, Gupta K, Tait C, Dalal I, Zhang C, Zhao E, Rustgi VK. COVID-19 status quo: Emphasis on gastrointestinal and liver manifestations. World J Gastroenterol. 2021;27:7969–7981. doi: 10.3748/wjg.v27.i46.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgakopoulou VE, Gkoufa A, Damaskos C, Papalexis P, Pierrakou A, Makrodimitri S, Sypsa G, Apostolou A, Asimakopoulou S, Chlapoutakis S, et al. COVID-19-associated acute appendicitis in adults. A report of five cases and a review of the literature. Exp Ther Med. 2022;24(482) doi: 10.3892/etm.2022.11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS-CoV-2 infection: A prospective cohort study. Gut. 2021;70:1925–1932. doi: 10.1136/gutjnl-2020-323800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cholongitas E, Bali T, Georgakopoulou VE, Giannakodimos A, Gyftopoulos A, Georgilaki V, Gerogiannis D, Basoulis D, Eliadi I, Karamanakos G, et al. Prevalence of abnormal liver biochemistry and its impact on COVID-19 patients' outcomes: A single-center Greek study. Ann Gastroenterol. 2022;35:290–296. doi: 10.20524/aog.2022.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, et al. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73:566–574. doi: 10.1016/j.jhep.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sobotka LA, Esteban J, Volk ML, Elmunzer BJ, Rockey DC. Acute liver injury in patients hospitalized with COVID-19. Dig Dis Sci. 2022;67:4204–4214. doi: 10.1007/s10620-021-07230-9. North American Alliance for the Study of Digestive Manifestation of COVID-19* [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekpanyapong S, Bunchorntavakul C, Reddy KR. COVID-19 and the liver: Lessons learnt from the EAST and the WEST, a year later. J Viral Hepat. 2022;29:4–20. doi: 10.1111/jvh.13590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein D, Roth N, Kim A, Epstein M, Hirschwerk D, Kvasnovsky CL, Satapathy SK. Presentation, patterns and predictive value of baseline liver tests on outcomes in COVID-19 patients without chronic liver disease. World J Gastroenterol. 2021;27:7350–7361. doi: 10.3748/wjg.v27.i42.7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gurala D, Al Moussawi H, Philipose J, Abergel JR. Acute liver failure in a COVID-19 patient without any preexisting liver disease. Cureus. 2020;12(e10045) doi: 10.7759/cureus.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gan Q, Gong B, Sun M, Cao Z, Zheng Y, Zhang Y, Wen P, Shen Y, Hong L, Hou T, et al. A high percentage of patients recovered from COVID-19 but discharged with abnormal liver function tests. Front Physiol. 2021;12(642922) doi: 10.3389/fphys.2021.642922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robles-Diaz M, Lucena MI, Kaplowitz N, Stephens C, Medina-Cáliz I, González-Jimenez A, Ulzurrun E, Gonzalez AF, Fernandez MC, Romero-Gómez M, et al. Use of Hy's law and a new composite algorithm to predict acute liver failure in patients with drug-induced liver injury. Gastroenterology. 2014;147:109–118. doi: 10.1053/j.gastro.2014.03.050. [DOI] [PubMed] [Google Scholar]
- 13.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini JR Jr, Sabbula B, Russe-Russe JR, Munshi RF, Meshoyrer D, Sajid N, Gutierrez A, Munnangi S, Szydziak E, Akella J. A retrospective analysis of COVID-19-infected patients with acute hepatitis who develop acute liver failure in a safety net hospital. BMJ Open Gastroenterol. 2021;8(e000738) doi: 10.1136/bmjgast-2021-000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melquist S, Estepp K, Aleksandrovich Y, Lee A, Beiseker A, Hamedani FS, Bassett J. COVID-19 presenting as fulminant hepatic failure: A case report. Medicine (Baltimore) 2020;99(e22818) doi: 10.1097/MD.0000000000022818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew M, Tang Z, Radcliffe C, Caruana D, Doilicho N, Ciarleglio MM, Deng Y, Garcia-Tsao G. Significant liver injury during hospitalization for COVID-19 is not associated with liver insufficiency or death. Clin Gastroenterol Hepatol. 2021;19:2182–2191. doi: 10.1016/j.cgh.2021.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paštrovic F, Lucijanic M, Atic A, Stojic J, Barisic Jaman M, Tjesic Drinkovic I, Zelenika M, Milosevic M, Medic B, Loncar J, et al. Prevalence and prognostic impact of deranged liver blood tests in COVID-19: Experience from the regional COVID-19 center over the cohort of 3812 hospitalized patients. J Clin Med. 2021;10(4222) doi: 10.3390/jcm10184222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krishnan A, Prichett L, Tao X, Alqahtani SA, Hamilton JP, Mezey E, Strauss AT, Kim A, Potter JJ, Chen PH, Woreta TA. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J Gastroenterol. 2022;28:570–587. doi: 10.3748/wjg.v28.i5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medetalibeyoglu A, Catma Y, Senkal N, Ormeci A, Cavus B, Kose M, Bayramlar OF, Yildiz G, Akyuz F, Kaymakoglu S, Tukek T. The effect of liver test abnormalities on the prognosis of COVID-19. Ann Hepatol. 2020;19:614–621. doi: 10.1016/j.aohep.2020.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.