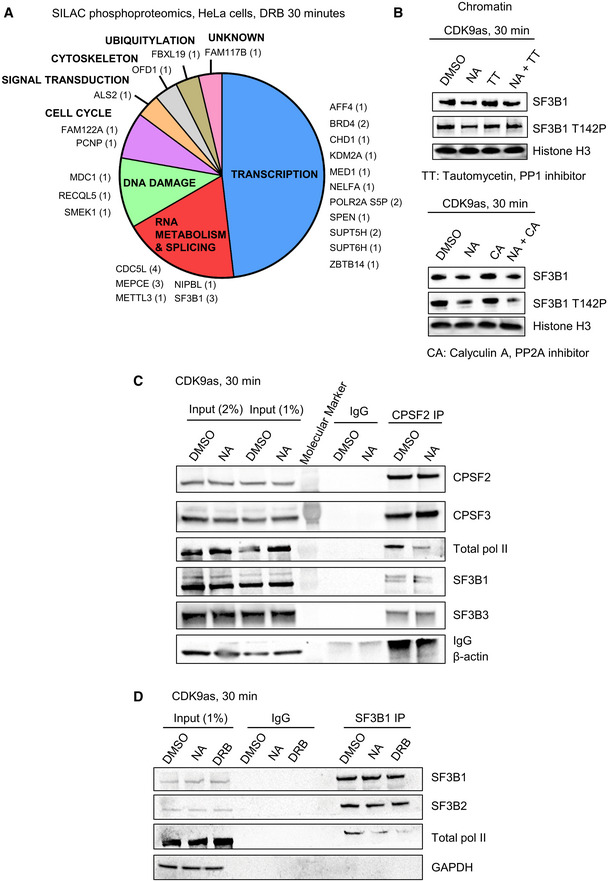
Figure 4. CDK9 phosphorylates several transcription and splicing factors in vivo and CDK9 inhibition promotes the loss of interaction of SF3B1 and CPA factors with pol II.
-
ADistribution of the proteins found to be phosphorylated by CDK9 in SILAC phosphoproteomics in HeLa cells treated or not with 100 μM DRB for 30 min (fold change > 1.5 in both biological replicates, number of phosphopeptides decreased shown in brackets).
-
BWestern blot of total SF3B1, SF3B1 T142P, and histone H3 as a loading control, on the chromatin fraction of CDK9as cells treated for 30 min with DMSO, NA, tautomycetin (TT), calyculin A (CA), NA + TT, or NA + CA. The histone H3 loading control (CA samples) is the same as the loading control shown in Appendix Fig S3B as the same Western blot experiment is shown in two different figs.
-
CCo‐immunoprecipitation of CPSF2 in the CDK9as cell treated for 30 min with DMSO or NA followed by Western blot with total pol II, CPSF2, CPSF3, SF3B1, SF3B3, and β‐actin (negative control) antibodies.
-
DCo‐immunoprecipitation of SF3B1 in the CDK9as cell treated for 30 min with DMSO, NA, or DRB followed by Western blot with total pol II, SF3B1, SF3B2, and GAPDH (negative control) antibodies.
