Figure 2. Interaction interfaces of the complex.
-
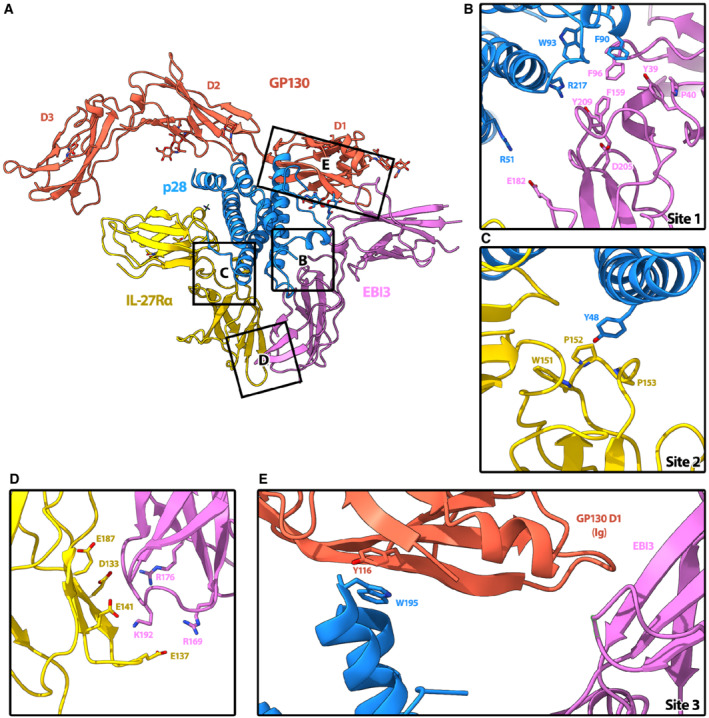
AOverview of IL‐27 interactions with signaling receptors. Ribbon representation of the complex colored according to protein components: p28 (blue), EBI3 (purple), IL‐27Rα (yellow), GP130 (red). Individual domains of GP130 (D1, D2, and D3) are labeled.
-
BThe hinge between the two CHR domains of EBI3 form a hydrophobic groove (Y39, P40, F96, F159, and Y209) that is filled by W93 of p28. Residue EBI3: D205, which is important for assembly of the heterodimeric cytokine (Rousseau et al, 2010), is also present in the binding interface and could form a salt bridge with p28:R217.
-
CIL‐27Rα binds site 2 of p28 at the apex of the elbow between its two CHR domains. The loops of IL‐27Rα form a pocket comprised of residues IL‐27Rα: W151, P152, and P153 in which the aromatic side chain of p28:Y48 could slot into.
-
DThe orientation of IL‐27Rα at site 2 is stabilized by a second interaction interface with EBI3, which is dominated by electrostatic complementarity between the two domains (IL‐27Rα: E187, D133, E141, E137, and EBI3:R176, R169, K192).
-
ESite 3 of p28 is occupied by the bend between the CHR domain 2 (D2) and the immunoglobulin (Ig) domain D1 of GP130. While this interface is not well resolved, p28:W195, which is essential for GP130‐mediated signaling (Rousseau et al, 2010), is facing D1. The key residues that mediate the interactions at interfaces are shown as sticks.
