Figure EV3. Doman structures of L(3)mbt, Lint‐O and their mutants and behavior of L(3)mbt‐S in OSCs.
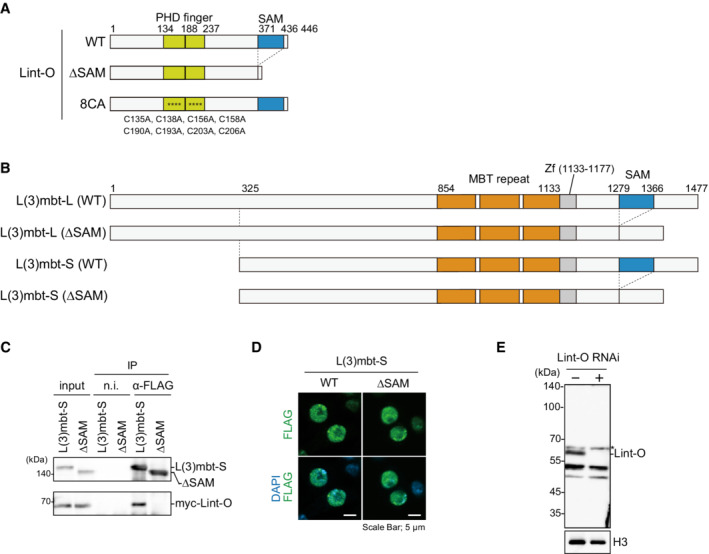
- Domain structures of WT Lint‐O and its ΔSAM and 8CA mutants. The ΔSAM mutant is composed of Met1‐Val371 and Ser437‐Asp446. The Cys‐to‐Ala mutations in the 8CA mutant are indicated at the bottom (asterisks in the structure).
- Domain structures of WT L(3)mbt‐L, WT L(3)mbt‐S, and their ΔSAM mutants. The ΔSAM mutant of L(3)mbt‐L is composed of Met1‐Leu1278 and Val1367‐Ser1477 of WT L(3)mbt‐L. The ΔSAM mutant of L(3)mbt‐S is composed of Met325‐Leu1278 and Val1367‐Ser1477 of WT L(3)mbt‐L.
- IP/western blotting shows that WT L(3)mbt‐S, but not its ΔSAM mutant, co‐immunoprecipitated with Lint‐O from the OSC lysates. n.i., nonimmune IgG.
- Subcellular localization of WT L(3)mbt‐S and its ΔSAM mutant (green). Scale bar: 5 μm.
- Western blotting using the anti‐Lint‐O antibodies raised in this study. The Lint‐O band (~60 kDa) was observed in normal OSCs (Lint‐O RNAi−) but not in Lint‐O‐depleted OSCs (Lint‐O RNAi+). Histone H3 (H3) was detected as a loading control. An asterisk shows the background.
Source data are available online for this figure.
