Figure7: L-DAP can interact with ASCT2 in several protonation states.
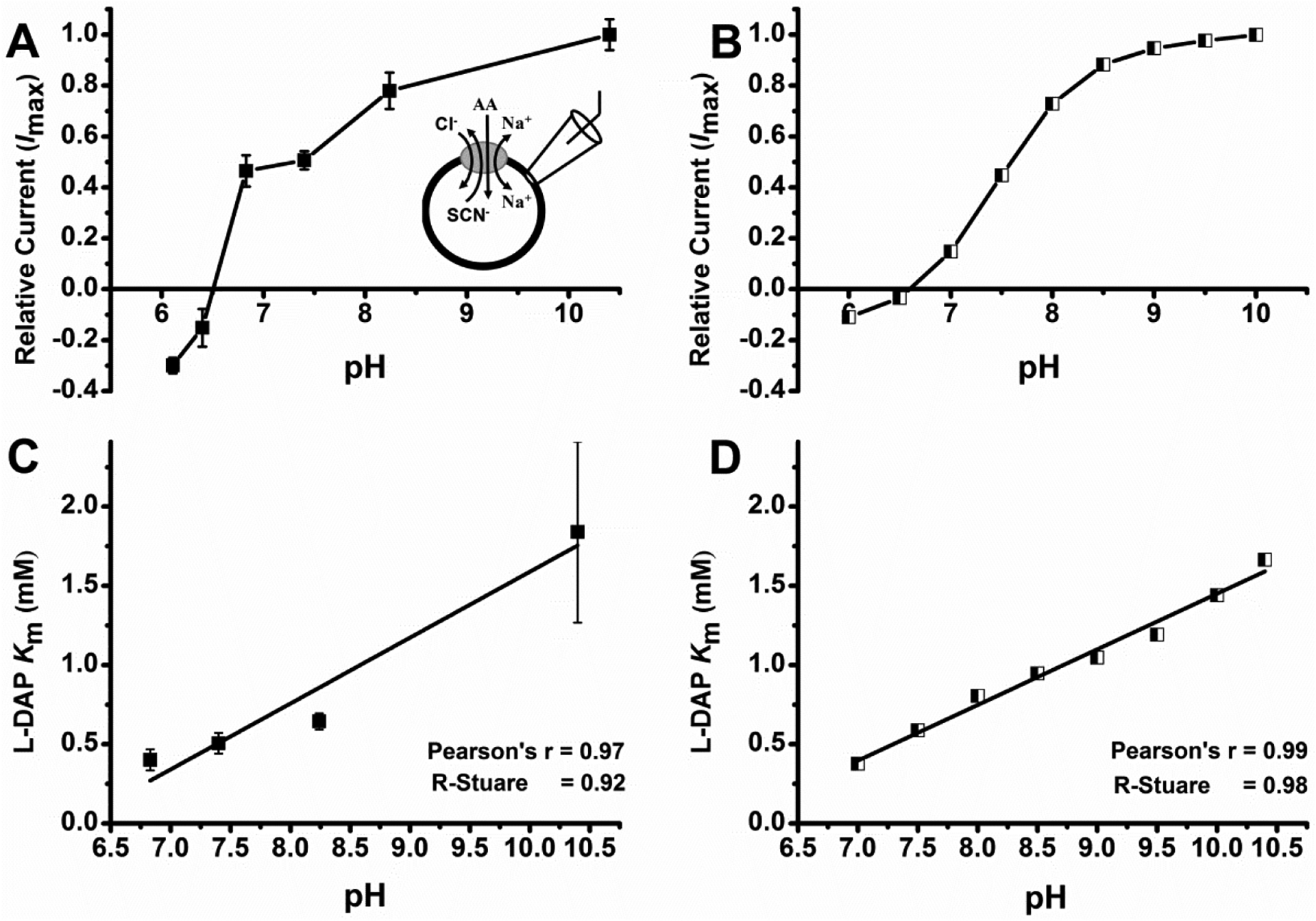
Determinationof pH dependence of L-Dap Km and relative currents. A, L-Dap relative current at different pH. L-DAP currents were normalized to the current response to 1 mM alanine at pH 7.4. Because alanine-induced currents are inward directed at pH 7.4, positive currents are inward, and negative currents are outward. B, calculated relative current vs. corresponding pH. pKa values for the transitions in eqn 1 were 6.8 and 9.8. Relative anion currents for states TA, TAH and TAH2 were 1.05, 0.45, and −0.28. The total anion current was calculated as I(total) = ITA[TA]+ ITAH[TAH]+ ITAH2[TAH2]. C, L-Dap binding affinity (Km) at different pH. D, Calculated L-DAP binding affinity at different pH. In all experiments, external bath solution was maintained at pH 7.40 and contained 140 mM NaCl and internal solution contained 140 mM NaSCN plus 10 mM alanine at pH 7.40. Running buffer, control and other solutions were at specified pH. All experiments were done at V = 0 mV.
