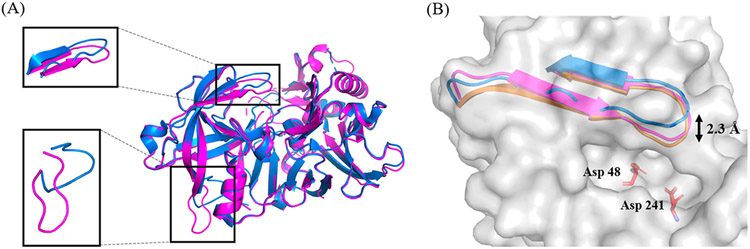
Figure 1.
(A) Overlay of BACE2 structures of 3ZKG (marine) and 6UJ0 (magenta, structure determined in this study). The boxed regions and associated insets show the enlargement of the overlay of flap-loop (top) and mobile loop102-110 (bottom). (B) Overlay of BACE2 flap-loop from different crystal forms. The BACE2 protein structure is shown in gray surface. The catalytic residues are shown in sticks and colored based on the atom types. The flap loops from different crystal forms are shown as ribbons. Structures for unbound BACE2 are shown for 6UJ0 (magenta, determined in this study) and 3ZKG (marine), as well as for inhibitor bound BACE2 (orange, PDB 2EWY). The double arrow indicates the observed flexibility of the flap-loop.