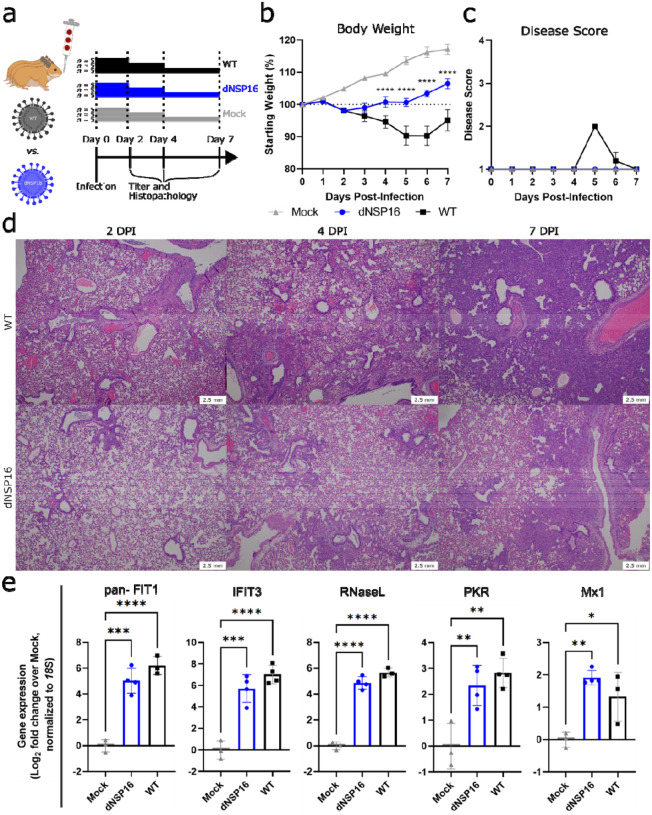
Figure 3. dNSP16 is attenuated in vivo.
(a) Overview of experimental plan for hamster infections. 100 μL inoculum of PBS (mock) or either dNSP16 (104 plaque-forming units) or WT (104 plaque-forming units) was given intranasally to 4- to 5-week-old Syrian hamsters. At 2, 4, and 7 days post-infection (DPI), 5 animals from each infection group were sacrificed for organ collection. Some graphics generated from BioRender. (b) Percent starting weights and (c) disease scores for mock-, dNSP16-, or WT-infected hamsters. ****p<0.001: results of a mixed-effects model (restricted maximum likelihood) with Tukey’s multiple comparison test (α = 0.05) performed between WT- and dNSP16-infected hamsters at the indicated DPI. Means are plotted with error bars denoting standard error of the mean. (d) Hematoxylin and eosin staining of representative 5 μm-thick sections taken from left lung lobes. (e) Fold change (log2) of expression of the indicated immune genes from right middle lung lobes isolated from hamsters infected with the indicated virus (or mock), 2 DPI. For each panel, fold changes from dNSP16 or WT samples are measured relative to mock samples. Values from individual hamsters are plotted (symbols) as well as means (bars). Error bars denote standard deviation. All samples were normalized to 18S expression, used as a reference. *p<0.05, **p<0.01, ***p<0.005, ****p<0.001: results of one-way ANOVA with Tukey’s multiple comparison test (α = 0.05).