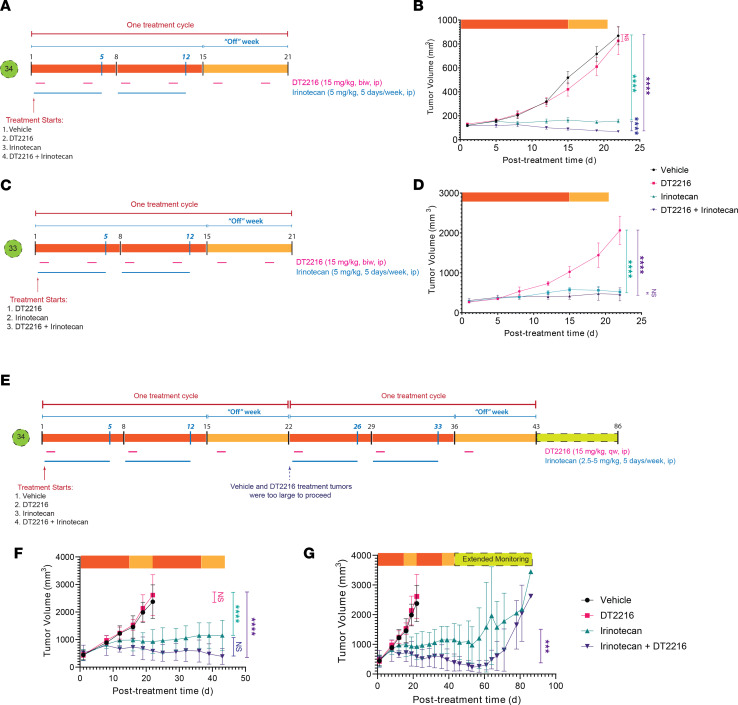
Figure 5. DTT216 administered i.p. twice a week combined with irinotecan.
(A and C) Illustration of treatment timeline for PDX 34 and PDX 33. DT2216 was administered i.p. twice a week for the entire treatment cycle; irinotecan was administered for 5 days a week for 2 weeks. The third week, the mice were off the treatment with irinotecan. (B and D) Changes in tumor volume over the course of treatment. Data are presented as the mean ± SEM (n = 7 for vehicle and irinotecan treatment groups, n = 6 for DT2216, n = 9 for other treatment groups at the start of the treatment for PDX 34, n = 3 for DT2216 and irinotecan, and n = 4 for the combo treatment at the start of treatment for PDX 33). ****P < 0.0001 in indicated comparisons. DT2216 with 2 cycles of treatment. (E) Illustration of treatment timeline for PDX 34. DT2216 was administered i.p. twice a week for the entire treatment cycle. A low dose of irinotecan (2.5 mg/kg) was administered i.p. for 5 days a week for the first treatment cycle, followed by an intermediate dose of irinotecan (5 mg/kg) administered for the second treatment cycle. The third week of each treatment cycle, the mice were off irinotecan. (F) Changes in tumor volume over the course of the treatment cycle. Data are presented as the mean ± SEM (n = 4 for all treatment groups at the start of the treatment for PDX 34). (G) Changes in tumor volume over the entire timeline (treatment cycle + extended monitoring beyond treatment). ***P < 0.001 in indicated comparisons, as determined by Linear mixed-effects regression model.