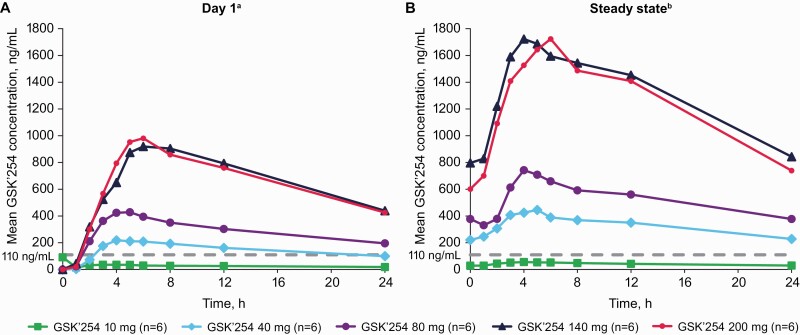
Figure 3.
Plasma GSK’254 PK parameters after (A) single-dose administration on day 1 and (B) repeat-dose administration at steady state. Dashed line indicates the clinical efficacy target value for which ≥95% of participants in a phase IIb study are projected to exceed target trough concentrations (110 ng/mL). Abbreviations: GSK’254, GSK3640254; PK, pharmacokinetics. aOne participant in the 10-mg group had a predose concentration that was inconsistent with the expected PK profile. One participant in the 200-mg group was excluded from PK analysis due to vomiting postdose ≤1 × time to maximum observed concentration. bSteady state was measured on day 8, 9, or 10 in part 1 and day 7 in part 2.