Abstract
Background:
Breast lumps have been reported as the most common breast symptom among adult females in Western Nigeria and are benign in 60% of cases. In South-Eastern Nigeria, fibroadenoma has been reported as the most common breast disease (47.5%), followed by carcinoma (30.4%) and fibrocystic disease. The aim of this study was to determine the correlation between sonographic and histopathologic findings in women who presented with breast masses.
Materials and Methods:
This was a cross-sectional study conducted among 160 consecutive female patients who presented with breast masses. A breast ultrasound scan was carried out to categorize the masses using the American College of Radiology Breast Imaging Reporting and Data System classification, and the histopathological diagnoses of the masses were obtained. The correlation of the sonographic findings and histopathological diagnoses was determined using the Statistical Package for Social Sciences (SPSS) IBM version 23.0.
Results:
Sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were found to be 79.5%, 98.3%, 93.9%, 93.7%, and 93.8%, respectively. There was a positive correlation between the sonographic findings and histopathological diagnoses of the breast masses, which was statistically significant (P = 0.000, r = 0.846).
Conclusion:
This study found a statistically significant positive correlation between sonographic findings and histopathological diagnoses of breast masses.
Keywords: BI-RADS, breast mass, histopathology, ultrasonography
Introduction
Breast disease in women encompasses a spectrum of benign and malignant disorders.[1] The most common breast problems for which women consult a physician are breast pain, nipple discharge, and a palpable mass.[1] Early menarche (before the age of 12),[2] late natural menopause (after the age of 55),[2] not bearing children, and first pregnancy over the age of 30 all increase lifetime exposure to oestrogen and progesterone and the risk of breast cancer.[2]
Diseases of the breast are very common in all age groups and encompass a spectrum of benign and malignant disorders.[1,3]
Masses are assessed by shape, margin, and density. The shape may be round, oval, irregular, or lobulated, and the margin may be smooth, obscured, indistinct, or spiculated. Benign lesions tend to be characterized as round or oval and well-defined, whereas malignant lesions tend to be irregular in shape and outline based on imaging.[4] Common causes of a benign breast mass include fibrocystic disease, fibroadenoma, cyst, galactocele, and abscess. Malignant breast disease encompasses many histologic types that include infiltrating and in-situ ductal or lobular carcinoma.[5]
Breast imaging which is the radiologic starting point for the assessment of breast findings plays a vital role in the multidisciplinary approach to the management of breast disease.[6] Symptomatic patients are evaluated by the triple assessment technique, which includes clinical breast examination (CBE), breast imaging such as mammography, breast ultrasound (BUS), and magnetic resonance imaging (MRI) as well as breast cytology or biopsy for histopathological diagnosis.[7]
Ultrasound is useful in the detection of breast masses as well as in the differentiation of masses seen on mammography and can detect lesions in younger women with dense breasts, as well as in pregnant and lactating women.[8,9] It is invaluable for interventional procedures and differentiation between benign and malignant breast masses and as such is an adjunct to clinical examination and mammography.[10,11,12,13]
Fine needle aspiration cytology, fine needle biopsy, core needle biopsy, or tru-cut biopsy and open surgical biopsy are the different methods of breast biopsy done to obtain tissue for histopathological diagnosis.[14]
The American College of Radiology (ACR) formulated the Breast Imaging Reporting and Data System (BI-RADS) to standardize breast imaging reporting.
Materials and Methods
This study was a prospective and cross-sectional study and conducted on 160 consecutive women attending the Surgical, Medical, and General outpatient clinics of Usmanu Danfodiyo University Teaching Hospital, Sokoto State, Nigeria. The study participants were females aged 16–75 years with breast masses seen during the study period. Male patients, patients who were unwilling to undergo the examination, patients with ulcerated/fungating mass in the breast, and those without a demonstrable breast mass were excluded from participating in the study. The sample size was calculated using the prevalence of breast cancer in Sokoto (10.4 per 100,000 women)[15] where consecutive patients with breast masses who presented for ultrasound scan were selected. Ethical approval was obtained from the Health Research Ethics Committee of the institution and has a protocol number of UDUTH/HREC/2016/No.523. Informed consent was obtained from each study participant. The research was conducted according to the principles of the Helsinki Declaration in dealing with human subjects in research. A structured questionnaire was used to collect the data on sociodemographic variables, clinical symptoms, sonographic findings, and histopathological outcome (benign or malignant) as well as histopathological diagnosis. The final assessment category was classified into four using BI-RADS 2 (benign), BI-RADS 3 (probably benign), BI-RADS 4 (suspicious of malignancy), and BI-RADS 5 (highly suggestive of malignancy). The ultrasound scan was done at the radiology department of the institution and carried out using the Mindray Digital Ultrasonic Diagnostic Imaging System having Doppler facility using the 7.5–10 MHz linear array transducer. The procedure was carried out according to the standard protocol for performing a breast ultrasound scan. The patients who were diagnosed with simple cysts had aspiration done with or without ultrasound guidance, whereas those with complex cysts and solid masses had biopsies taken either by ultrasound guidance or open biopsy. Sixty-eight study participants had ultrasound-guided biopsy done, whereas the remaining 92 patients had open biopsy. The images were acquired, saved on the machine, and printed out when required using the Mitsubishi printer attached to the ultrasound machine. The histopathologic diagnoses were obtained thereafter. Sonographic assessment by BI-RADS classification was evaluated against histopathological outcomes and signal detection theory applied to identify true-positive, true-negative, false-positive, and false-negative examinations. All findings and measurements were documented in the questionnaire under the appropriate section.
Data were analysed using the Statistical Package for Social Sciences (SPSS) version 23 for Windows (IBM, Chicago, IL, USA). Analysis began with descriptive statistics using mean and standard deviation for quantitative data and frequency, as well as percentages for qualitative data. The χ2 test was used to determine associations between categorical variables. The results were presented in the form of texts and tables. The level of statistical significance was set at P < 0.05 at 95% confidence interval. The correlation coefficients, sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy were also determined. Some of the limitations encountered in the study include bias in ultrasound scan findings and a few cases of inadequate samples as well as wrong tissue sampling. Ultrasound findings were crosschecked by another radiologist and sample re-collection was done to reduce error.
Results
A total of 160 female participants completed the study. The age of the patients ranged between 16 and 75 years, with a mean of 33.03 ± 12.32 years. Most of the participants 82 (51.2%) were in the 15–30 years age group. One hundred and twelve (70%) were of Hausa/Fulani ethnicity, and more than half [102 (63.7%)] were unemployed [Table 1].
Table 1.
Sociodemographic characteristics of study subjects
| Variab les | N | (%) | |
|---|---|---|---|
| Age (years) | 15–30 | 82 | 51.2 |
| 31–45 | 58 | 36.3 | |
| 46–60 | 15 | 9.4 | |
| 61–75 | 5 | 3.1 | |
| Tribe | Hausa/Fulani | 112 | 70.0 |
| Yoruba | 17 | 10.6 | |
| Igbo | 12 | 7.5 | |
| Others | 19 | 11.9 | |
| Religion | Islam | 135 | 84.4 |
| Christianity | 24 | 15.0 | |
| Others | 1 | 0.6 | |
| Educational qualification | None | 12 | 7.5 |
| Quranic | 11 | 6.9 | |
| Primary | 5 | 3.1 | |
| Secondary | 66 | 41.2 | |
| Graduate | 62 | 38.8 | |
| Postgraduate | 4 | 2.5 | |
In line with the study, all the patients presented with breast lump/mass. Table 2 shows the distribution of clinical symptoms, and 46 (28.7%) patients had associated breast pain, whereas only 8 (5.0%) had associated nipple discharge. The findings were commoner on the right side [83 (51.9%)].
Table 2.
Distribution of clinical symptoms of the patients
| Variables | N | (%) | |
|---|---|---|---|
| Presence of a right breast mass | Yes | 83 | 51.9 |
| No | 77 | 48.1 | |
| Presence of a left breast mass | Yes | 77 | 48.1 |
| No | 83 | 51.9 | |
| Presence of associated pain | Yes | 46 | 28.7 |
| No | 114 | 71.3 | |
| Presence of nipple discharge | Yes | 8 | 5.0 |
| No | 152 | 95.0 | |
| Type of nipple discharge | Serous/milky | 4 | 50.0 |
| Bloody | 3 | 37.5 | |
| Brownish | 1 | 12.5 | |
Thirty-two (20.0%) had multiple masses in the breast, with 26 (16.3%) noted in more than one quadrant. More than half of the masses were oval in shape, wider than tall with circumscribed margin. Final BI-RADS assessments were categorized into benign (BI-RADS 2), probably benign (BI-RADS 3), suspicious of malignancy (BI-RADS 4), and highly suggestive of malignancy (BI-RADS 5), which were found to constitute 65%, 14.4%, 11.3%, and 9.4%, respectively [Table 3].
Table 3.
Distribution of sonographic findings and final BI-RADS assessment
| Variables | N | (%) | |
|---|---|---|---|
| Number of breast | Single | 128 | 80.0 |
| masses | Multiple | 32 | 20.0 |
| Location of the mass (quadrants) | Upper inner | 48 | 30.0 |
| Upper outer | 44 | 27.5 | |
| Lower inner | 24 | 15.0 | |
| Lower outer | 18 | 11.3 | |
| Multiple | 26 | 16.3 | |
| Shape of the mass | Oval | 127 | 79.4 |
| Round | — | — | |
| Irregular | 33 | 20.6 | |
| Orientation of the mass | Taller than wide | 34 | 21.3 |
| Wider than tall | 126 | 78.8 | |
| Margin of the mass | Circumscribed | 123 | 76.9 |
| Not circumscribed | 37 | 23.1 | |
| Echopattern of the mass | Anechoic | 41 | 25.6 |
| Hypoechoic | 94 | 58.8 | |
| Isoechoic | — | — | |
| Hyperechoic | 20 | 12.5 | |
| Heterogeneous | 5 | 3.1 | |
| Presence of architectural distortion | Yes | 21 | 13.1 |
| No | 139 | 86.9 | |
| Final BI-RADS | 2 | 104 | 65.0 |
| category | 3 | 23 | 14.4 |
| 4 | 18 | 11.3 | |
| 5 | 15 | 9.4 | |
Table 4 shows the histological outcome with benign masses accounting for 121 (75.6%) and malignant masses accounting for 39 (24.4%). The most common benign diagnosis was fibroadenoma showing circumscribed margin and lateral shadowing [sonographic and histopathological images as shown in Figure 1] constituting 79 patients (49.4%), whereas invasive ductal carcinoma (IDC) [21 (13.1%)] was the commonest malignant lesion.
Table 4.
Distribution of histopathological outcome and diagnosis of the breast masses
| Variables | N | (%) | |
|---|---|---|---|
| Nature of mass (outcome) | Benign | 121 | 75.6 |
| Malignant | 39 | 24.4 | |
| Histopathological diagnosis | Fibroadenoma | 79 | 49.4 |
| • Benign | Fibrocystic changes | 6 | 3.8 |
| • Malignant | Cyst | 14 | 8.8 |
| Abscess (inflammatory cells) | 14 | 8.8 | |
| Intramammary lymph node | 1 | 0.6 | |
| Lipoma | 2 | 1.3 | |
| Galactocele | 3 | 1.9 | |
| Duct ectasia/hyperplasia | 2 | 1.3 | |
| Invasive ductal carcinoma | 21 | 13.1 | |
| Ductal carcinoma in-situ | 14 | 8.8 | |
| Malignant phyllodes tumour | 1 | 0.6 | |
| Mucinous (colloid) adenocarcinoma | 1 | 0.6 | |
| Invasive lobular carcinoma | 2 | 1.3 | |
Figure 1.
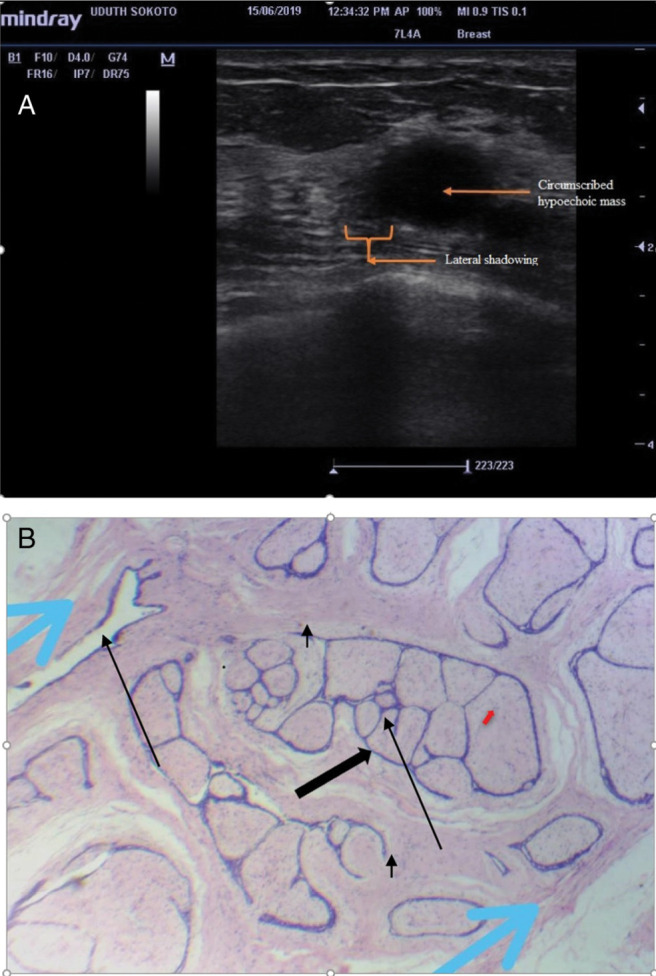
(a) Sonographic image of a right breast mass in a 41-year-old P3+0 showing a circumscribed hypoechoic mass with lateral shadowing. (b) Histology section confirmed the diagnosis of fibroadenoma. The section shows proliferating breast glands and the glands are compressed into slit-like spaces (black arrow [arrow], stroma [red arrows], and capsule [blue arrow])
Majority of the study participants with benign mass [78 (64.5%)] were within the 15–30 years age group, and 101 (83.5%) had benign outcome on histology with BI-RADS category of 2 (benign finding) on ultrasound. This was statistically significant (P = 0.000) [Table 5].
Table 5.
Relationship between sonographic findings and the histopathological outcome
| Nature on histopathology | Test statistics and P-value | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Benign | Malignant | |||||
|
|
|
|||||
| N | % | N | % | |||
| Age group (years) | 15–30 | 78 | 64.5 | 4 | 10.3 | *χ2=48.022, P=0.000 |
| 31–45 | 38 | 31.4 | 20 | 51.3 | ||
| 46–60 | 4 | 3.3 | 11 | 28.2 | ||
| 61–75 | 1 | 0.8 | 4 | 10.3 | ||
| Ultrasound BI-RADS category | 2 | 101 | 83.5 | 3 | 7.7 | χ2=113.323, P=0.000 |
| 3 | 18 | 14.9 | 5 | 12.8 | ||
| 4 | 2 | 1.7 | 16 | 41.0 | ||
| 5 | 0 | 15 | 38.5 | |||
*χ2 = Fisher’s exact test
There was a strong significant positive correlation between sonographic and histopathological findings of breast masses (r = 0.826; P < 0.000). The sensitivity, specificity, PPV, NPV, and accuracy for the correlation of the sonographic findings and histopathological diagnoses were found to be 79.5%, 98.3%, 93.9%, 93.7%, and 93.8% respectively, all at 95% confidence interval [Table 6].
Table 6.
Distribution of sensitivity, specificity, PPV, NPV, and accuracy for sonographic and histopathological correlation of the breast masses
| Variables | Sensitivity (%), 95% CI | Specificity (%), 95% CI | PPV (%), (%), 95% CI | NPV (%), (%), 95% CI | Accuracy (%), (%), 95% CI | Test statistics and P-value |
|---|---|---|---|---|---|---|
| Sonography correlation with histopathology (r = 0.826) | 79.5 | 98.3 | 93.9 | 93.7 | 93.8 | χ2 = 109.143, P = 0.000 |
PPV = positive predictive value, NPV = negative predictive value, CI = confidence interval, χ2 = chi-square test, P= P-value
*Correlation is significant at P<0.05 (two-tailed), r = correlation coefficient value
Discussion
Breast cancer is one of the leading causes of death in women.[16] With growing awareness in the general population about breast cancer, the presence of a breast mass causes anxiety.[17]
Breast sonography is the examination of choice in the young and for dense breast as it is safe, dynamic, and does not use ionizing radiation. Early identification of malignant features by high-frequency ultrasound reduces morbidity and improves the overall management.[9] Histology of breast masses has been identified as the confirmatory test and the gold standard for diagnosis.[18]
The ACR formulated the BI-RADS to standardize breast imaging reporting to avoid ambiguity in communication and interpretation.[19,20,21] The need for standardization of reports of breast ultrasound and mammography in Nigeria has been reviewed by Obajimi et al.[10] in Ibadan and Akhigbe and Omuemu,[21] respectively.
In this study, the age of the participants ranged from 16 to 75 years with a mean age of 33.03 ± 12.32 years which is similar to what was obtained in the studies by Chandak and Dhande[5] and Obajimi et al.,[10] in which the age range of patients and mean age were 11–70 years and 38.54 years and 14–74 years and 38.91 ± 12.51 years, respectively.
Most (51.9%) of the breast masses in this study were found in the right breast. On the contrary, Muddegowda et al.[3] in India found more masses in the left breast (74.66%).
This study found that majority of the masses 48/160 and 44/160 were located in the upper outer and upper inner quadrants, respectively. This finding was similar to a great extent to the study on “Evaluation of breast masses by sonomammography and X-ray mammography” by Chandak and Dhande,[5] who also found that most of the lesions in their study were found in the upper quadrants. However, the masses were slightly more in the inner (upper inner) 21/50 than in the outer (upper outer) 19/50 quadrants.
This study showed the most common sonographic features for benign masses to be oval shape 98.4%, parallel (wider than tall) orientation with 96.9%, and circumscribed margin 96.1%. The features for malignant masses, on the contrary, were irregular shape and anti-parallel (taller than wide) orientation with 76.9% each, and not circumscribed margin 79.5%. When Pearson’s correlation was applied, it showed statistically significant relationship between sonographic findings and histopathological diagnoses. This correlation showed that shape, orientation, and mass margin are good and reliable descriptors to predict either benignity or malignancy. The findings in this study are comparable to the findings by Okeji et al.,[18] who described common ultrasound features for benign lesions to be oval shape (58.5%), a parallel orientation (85.1%), and well-circumscribed margin (89.4%). Chandak and Dhande[5] also noted that ellipsoid shape, wider than tall orientation, posterior enhancement with lateral shadowing are good predictors of benignity, whereas microlobulations were described as good predictors for malignancy. In the study by Sudheer,[22] benign lesions were described to be well-defined; oval or round; anechoic, isoechoic, or hypoechoic depending on the lesion; and wider than tall. They described malignant lesions as commonly hypoechoic with ill-defined borders, taller than wide, having spiculated margins, posterior acoustic shadowing, and microcalcifications.
In this study, it was found that most of the benign lesions were hypoechoic and anechoic in nature, 63% and 32.3%, respectively, and this was found to be statistically significant (P = 0.000). However, Okeji et al.[18] found hyperechoic masses to be more related to benignity than hypoechoic masses. They found hyperechoic and anechoic echopatterns to be features for benignity in 43.6% and 37.2%, respectively. Chandak and Dhande[5] concluded in their study that even though marked hypoechogenicity is a worrisome finding for malignancy, isoechogenicity/mild hypoechogenicity is not necessarily reassuring and should be considered as indeterminate findings. This may also be explained by methodological differences and disparity of facilities used in the study areas.
Generally, benign breast diseases are commoner than their malignant counterparts,[3,16,23] and fibroadenoma has been shown to be the commonest histologically proven benign breast disease.[3,16]
In this study, fibroadenoma was found in 49.4% of the patients and had the highest occurrence, followed by cysts (8.8%) and abscesses (8.8%). Okoye and co-workers[23] in their findings, which is similar to this study and that of Muddegowda et al.,[3] found that benign breast lesions occurred more frequently. Okoye and co-workers[23] and Muddegowda et al.[3] found that fibroadenoma was also the most common benign breast lesion followed by fibrocystic diseases. Naz and Malik[9] found more cysts (37.5%) than fibroadenoma (28.5%) in their study.
IDC was the most common malignant breast lesion followed by ductal carcinoma in-situ (DCIS) in this study. The diagnostic outcome in the index study was similar to the findings of Mustapha et al.[24] and Muddegowda et al.,[3] which showed that IDC was also the commonest malignant lesion identified in their studies, respectively.
In this study, the ratio of benign to malignant breast lesions was found to be 3.1:1. Okoye and co-workers[23] in their study found that benign breast lesions occurred more frequently than malignant breast lesions with a ratio of 2.3:1; they also found that benign lesions tend to occur 20 years earlier than the malignant lesions.
The oldest patient to have a benign lesion in this study was 75 years. This is similar to the study by Chandak and Dhande,[5] who found the oldest patient to have a benign lesion to be 60 years of age. The youngest patient with breast malignancy was 30 years of age which is also similar to the finding by Chandak and Dhande,[5] in which they found the youngest patient in their study with malignant lesion to be 31 years of age. The latter finding is in line with the findings by Chen et al.[25] that the African American woman is likely to be diagnosed with an aggressive variant of breast cancer at a much younger age based on the impact of race and ethnicity on breast cancer.
Correlation between sonographic findings and histopathological diagnoses was found to be statistically significant (P = 0.000), with r= 0.846. The sensitivity, specificity, PPV, NPV, and accuracy of the index study were found to be 79.5%, 98.3%, 93.9%, 93.7%, and 93.8%, respectively. A similar finding was obtained in the study by Adeyomoye et al.[26] when histology was correlated with ultrasound, giving the overall sensitivity of diagnostic ultrasound as 83.9%, while PPV, specificity, NPV, and accuracy were 96.3%, 99.2%, 96.1%, and 96.1%, respectively. Adeyomoye et al.[27] consequently advocated the use of imaging in conjunction with needle biopsy to achieve improved sensitivity and to avoid unnecessary benign surgical biopsies.
Conclusion
This study found a significant positive correlation between the sonographic findings and histopathological diagnoses of breast masses. The coefficient of correlation of 0.846 was found in this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Monica M. Evaluation of common breast problems. Am Fam Phys. 2000;61:2371–8. [PubMed] [Google Scholar]
- 2.McPherson K, Steel CM, Dixon JM. ABC of breast diseases. Br Med J. 2000;321:624–8. doi: 10.1136/bmj.321.7261.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muddegowda PH, Lingegowda JB, Kurpad R, Konapur P, Shivarudrappa A, Subramaniam P. The value of systematic pattern analysis in FNAC of breast lesions: 225 cases with cytohistological correlation. J Cytol. 2011;28:13–9. doi: 10.4103/0970-9371.76942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitley AS, Sloane C, Hoadley G, Moore AD, Alsop CW. London: Hodder Arnold; 2005. Mammography: Clark’s Positioning in Radiography: Hodder Arnold Twelfth edition; pp. 436–41. 445-63. [Google Scholar]
- 5.Chandak NS, Dhande R. Evaluation of breast masses by sonomammography and X-ray mammography in correlation with histopathological findings. Int J Recent Surg Med Sci. 2017;3:3–6. [Google Scholar]
- 6.Akande HJ, Olafimihan BB, Oyinloye OI. A five year audit of mammography in a tertiary hospital north central Nigeria. Niger Med J. 2015;56:213–7. doi: 10.4103/0300-1652.160401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ACR® American College of Radiology. 2015. [[Last accessed on Jul 5, 2019]]. Available from: https://www.acr.org>2015-Press-release .
- 8.Adam A, Dixon AK. Grainger and Allison's diagnostic radiology. A Textbook of Medical Imaging. 5th ed. London: Elsevier (Churchill Livingstone); 2008. pp. 1173–8. [Google Scholar]
- 9.Naz N, Malik K. Different appearances of breast masses on high frequency ultrasound. Pak J Surg. 2012;28:65–9. [Google Scholar]
- 10.Obajimi MO, Adeniji-Sofoluwe AT, Adedokun BO, Soyemi TO, Bassey OS. Sonographic breast pattern in women in Ibadan Nigeria. Ann Afr Med. 2014;13:145–50. doi: 10.4103/1596-3519.142269. [DOI] [PubMed] [Google Scholar]
- 11.Rahul S, Manjula HV, Ashok K. Step by Step Breast Ultrasound. 1st ed. New Delhi: Jaypee Medical Publishers Ltd; 2006. pp. 4–5. 38-9, 52, 56, 62-75. [Google Scholar]
- 12.Kohn TM, Lichy J, Newhouse JH. Comparison of the performance of the screening mammography physical examination and breast ultrasound and evaluation of the factors that influence them. Radiology. 2012;235:165–75. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 13.Freitas SLC, Furtado JXA. Correlation between ultrasonographic features and histopathologic findings of breast lesions in biopsies. Mastology. 2017;27:225–9. [Google Scholar]
- 14.Obajimi MO, Adeniji-Sofoluwe AT, Soyemi TO, Oluwasola AO, Afolabi AO, et al. Ultrasound-guided core biopsy of breast lesions in Ibadan: Our initial experience. J Clin Sci. 2015;12:3–8. [Google Scholar]
- 15.Agbo PS, Oboirien M, Gana G. Breast cancer incidence in Sokoto Nigeria. Int J Dev Sustain. 2013;2:1614–22. [Google Scholar]
- 16.Parkin DM, Bray FJ, Devessa SS. Cancer burden in year 2000. Eur J Cancer. 2001;37:54–66. doi: 10.1016/s0959-8049(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 17.Ahmed I, Nazir R, Chaudhary MY, Kundi S. Triple assessment of breast lump. J Coll Phys Surg Pak. 2007;17:535–8. [PubMed] [Google Scholar]
- 18.Okeji MC, Agwu KK, Agwuna KK, Nwachukwu IC. Sonographic features and its accuracy at differentiating between benign and malignant breast lesions in Nigerian women. World J Med Sci. 2015;12:370–4. [Google Scholar]
- 19.ACR BI-RADS Atlas. Breast Imaging Reporting and Data System. (5th ed) 2013 [Google Scholar]
- 20.Stavros AT, Thickman D, Rapp CL, Dennis MA, Parker SH, Sisney GA. Solid breast nodules: Use of sonography to distinguish between benign and malignant lesions. Radiology. 1995;196:123–34. doi: 10.1148/radiology.196.1.7784555. [DOI] [PubMed] [Google Scholar]
- 21.Akhigbe AO, Omuemu VO. Knowledge attitudes and practice of breast cancer screening among female health workers in a Nigerian urban city. BMC Cancer. 2009;9:203. doi: 10.1186/1471-2407-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudheer G. Ultrasound characterization of breast masses. Indian J Radiol Imaging. 2009;19930:242–7. doi: 10.4103/0971-3026.54878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anyikam A, Nzegwu MA, Ozumba BC, Okoye I, Olusina DB. Benign breast lesions in Eastern Nigeria. Saudi Med J. 2008;29:241–4. [PubMed] [Google Scholar]
- 24.Mustapha Z, Abubakar A, Modu AA, Pindiga UH, Okedayo M, et al. Histological outcome of BIRADS 5 breast lesions in Maiduguri, North Eastern Nigeria. Borno Med J. 2014;11:119–122. [Google Scholar]
- 25.Chen Z, Wu AH, Gauderman WJ, Bernstein L, Ma H, Pike MC, et al. Does mammographic density reflect ethnic differences in breast cancer incidence rates? Am J Epidemiol. Am J Epidemiol. 2004;159:140–7. doi: 10.1093/aje/kwh028. [DOI] [PubMed] [Google Scholar]
- 26.Adeyomoye AAO, Awosanya GOG, Osibogun A. Performance measures of diagnostic ultrasound of the breast in Lagos University Teaching Hospital (LUTH) Nigeria. Nigerian Med Practitioner. 2006;49:28–33. [Google Scholar]
- 27.Adeyomoye AAO, Awosanya GOG, Adesanya AA, Anunobi CC, Osinbogun A. Medical audit of diagnostic mammographic examination of the Lagos University Teaching Hospital (LUTH) Nigeria. Niger Postgrad Med J. 2009;16:25–30. [PubMed] [Google Scholar]


