Fig. 1.
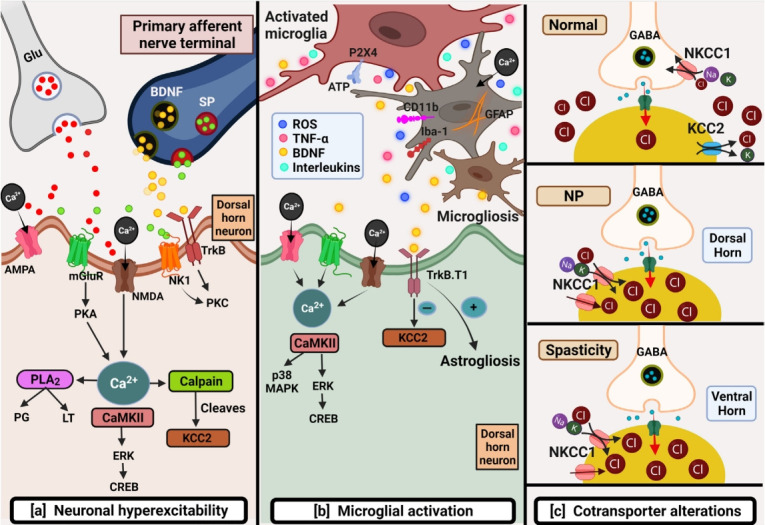
Schematic depicting primary mechanisms involved in chronic neuropathic pain post-spinal cord injury (SCI). (A) Neuronal hyperexcitability: several factors such as the excessive release of excitatory amino acids like glutamate and downregulation of glutamate transporters cause overactivation of glutamate receptors leading to glutamate excitotoxicity post-SCI. Glutamatemediated sustained depolarization causes heavy calcium influx promoting several downstream pathways responsible for hypoactive inhibitory tone thus producing ongoing pain. (B) Microglial activation: This can be identified by the expression of several markers like CD11b, GFAP, P2X4, and Iba-1 post-SCI. Microglia further by initiating downstream pathways promote microgliosis and astrogliosis responsible to maintain inflammation-mediated pain. (C) Cotransporter alterations: SCI brings alterations in cotransporters that maintain chloride homeostasis. Normally, NKCC1 by Cl- influx, and KCC2 by Cl- efflux maintain Cl- concentration balance. Upregulation of NKCC1 and downregulation of KCC2 in dorsal and ventral horn are responsible for NP and spasticity post-SCI respectively AMPA, alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid; BDNF, brain derived neurotrophic factor; CREB, cAMP response element-binding protein; ERK, extracellular regulated kinase; Glu, glutamate; KCC2, potassium (K+)/chloride (Cl-) cotransporter; MAPK, mitogen-activated protein kinase; mGluR, metabotropic glutamate receptor; NK1, neurokinin 1 receptor; NKCC1, Na+-K+-Cl- cotransporter; NMDA, N-Methyl-D-aspartate; NP, neuropathic pain; PKA, cAMP-dependent protein kinase A; PKC, protein kinase C; PLA2, phospholipase A2; ROS, reactive oxygen species; SP, substance P; TNFα, tumor necrosis factor alpha; TrkB, tropomyosin receptor kinase B.
