The COVID‐19 pandemic has had a profound impact on healthcare services worldwide due to the redeployment of staff and suspension of many scheduled medical and surgical activities. 1 In the context of skin malignancies, treatment regimens have been modified to decrease the need for hospital visits. Various studies have suggested that neglecting skin cancers (i.e. melanoma and non‐melanoma skin cancers) throughout the COVID‐19 outbreak will be associated with increased rates of morbidity, mortality and healthcare expenses. 2 , 3 We therefore aimed to assess the safety and reliability of pathways followed to deliver SACT to patients with skin cancers during the first wave of the COVID‐19 pandemic, as to inform other cancer centres which are currently developing guidelines to provide safe SACT for skin cancer.
From the onset of the COVID‐19 pandemic, Guy's Cancer Centre restructured its patient and staff/consultation pathway to provide cancer care while diminishing the risk of exposure to COVID‐19 (Figure 1). Using descriptive statistics, we analysed the socio‐demographic, clinical and COVID‐19 outcomes of skin cancer patients who received SACT between 1 March 2020 and 31 May 2020 (compared with the same period in 2019).
FIGURE 1.
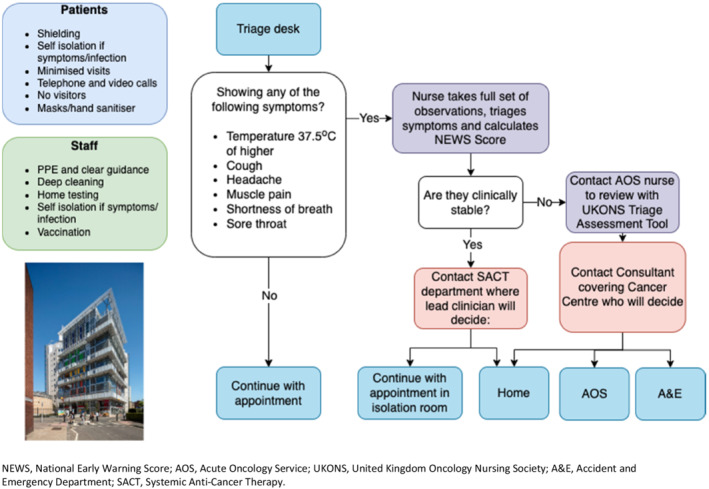
COVID‐19 Patient Pathway at Guy's Cancer Centre NEWS, National Early Warning Score; AOS, Acute Oncology Service; UKONS, United Kingdom Oncology Nursing Society; A&E, Accident and Emergency Department; SACT, Systemic Anti‐Cancer Therapy
Clinical and demographic characteristics of these cancer patients are summarized in Table 1. We observed an increase of 20% in the total number of SACT administered for skin cancers in 2020 (n = 112), in comparison to 2019 (n = 92). During this period, only one skin cancer patient receiving SACT tested positive for COVID‐19. There was a significant increase (20%) in the number of immunotherapies administered in 2020 compared with 2019. This is in line with previous studies including the UK‐based consensus guidelines where the authors advised to continue prioritizing first‐line use of immunotherapy (single agent or combined) for metastatic skin cancer during the COVID‐19 pandemic. 4
TABLE 1.
Patient characteristics of skin cancer patients receiving systemic anti‐cancer treatment between 1 March 2020 and 31 May in 2019 and 2020
| Systemic anti‐cancer treatment patients (n = 359) | Systemic anti‐cancer treatment | p‐Value | |||
|---|---|---|---|---|---|
| 2019 (n = 92) | 2020 (n = 112) | ||||
| +20% | |||||
| n | % | n | % | ||
| Sex | |||||
| Male | 53 | 57.60% | 57 | 50.90% | 0.16 |
| Female | 39 | 42.40% | 55 | 49.10% | 0.16 |
| Age | |||||
| <50 | 18 | 19.60% | 30 | 26.80% | 0.10 |
| 50–59 | 17 | 18.50% | 24 | 21.40% | 0.29 |
| 60–69 | 21 | 22.80% | 21 | 18.80% | 0.23 |
| 70–79 | 17 | 18.50% | 27 | 24.10% | 0.16 |
| ≥80 | 19 | 20.70% | 10 | 8.90% | 0.00 |
| Mean (SD) | 64.6 | 15.9 | 60.5 | 15.1 | |
| Socio‐economic status | |||||
| Low | 64 | 69.60% | 91 | 81.20% | 0.02 |
| Medium | 10 | 10.90% | 5 | 4.50% | 0.04 |
| High | 14 | 15.20% | 14 | 12.50% | 0.28 |
| Missing | 3 | 3.30% | 2 | 1.80% | 0.25 |
| Ethnicity | |||||
| White British | 59 | 64.10% | 61 | 54.50% | 0.07 |
| White Other | 10 | 10.90% | 14 | 12.50% | 0.35 |
| Black African | 0 | 0.00% | 2 | 1.80% | |
| Asian | 0 | 0.00% | 1 | 0.90% | 0.07 |
| Other | 0 | 0.00% | 1 | 0.90% | |
| Unknown | 23 | 25.00% | 33 | 29.50% | 0.15 |
| Performance status | |||||
| 0 | 44 | 47.80% | 45 | 40.20% | 0.13 |
| 1 | 38 | 41.30% | 41 | 36.60% | 0.24 |
| 2 | 7 | 7.60% | 3 | 2.70% | 0.05 |
| 3 | 1 | 1.10% | 0 | 0.00% | 0.15 |
| Missing | 2 | 2.20% | 23 | 20.50% | 0.00 |
| Stage | |||||
| 1 | 0 | 0.00% | 0 | 0.00% | |
| 2 | 1 | 1.10% | 1 | 0.90% | 0.44 |
| 3 | 7 | 7.60% | 42 | 37.50% | 0.00 |
| 4 | 75 | 81.50% | 69 | 61.60% | 0.00 |
| Missing | 9 | 9.80% | 0 | 0.00% | 0.00 |
| Systemic anti‐cancer therapy type | |||||
| Chemotherapy | 7 | 7.60% | 3 | 2.70% | 0.05 |
| Immunotherapy | 40 | 43.50% | 71 | 63.40% | 0.00 |
| Biological/targeted | 39 | 42.40% | 33 | 29.50% | 0.02 |
| Combo biological/targeted | 2 | 2.20% | 2 | 1.80% | 0.42 |
| Combined Chemo | 3 | 3.30% | 0 | 0.00% | 0.03 |
| Combo IO | 1 | 1.10% | 3 | 2.70% | 0.19 |
| Treatment Paradigm | |||||
| Neoadjuvant | 0 | 0.00% | 1 | 0.90% | 0.15 |
| Adjuvant | 2 | 2.20% | 35 | 31.20% | 0.00 |
| Radical | 0 | 0.00% | 6 | 5.40% | 0.00 |
| Palliative | 90 | 97.80% | 69 | 61.60% | 0.00 |
| Curative | 0 | 0.00% | 1 | 0.90% | 0.15 |
| Line of Palliative Treatment (2019, n = 355; 2020, n = 274) | |||||
| 1 | 0 | 0.00% | 0 | 0.00% | |
| 2 | 49 | 53.30% | 48 | 42.90% | 0.06 |
| 3 | 22 | 23.90% | 16 | 14.30% | 0.04 |
| 4 | 5 | 5.40% | 3 | 2.70% | 0.16 |
| 5 | 1 | 1.10% | 0 | 0.00% | 0.15 |
| Missing | 0 | 0.00% | 1 | 0.90% | 0.15 |
| Overall mortality at 6 months | |||||
| 4 | 4.34% | 3 | 2.67% | ||
Furthermore, there was a 10% decrease in the total number of targeted therapies. However, patients undergoing targeted treatment were already being seen less often, in comparison with other SACT patients, before the start of the COVID‐19 pandemic. Therefore, this decrease in total number of therapies may not necessarily be linked with the pandemic. When looking at the treatment paradigm, there was an exponential rise in the number of adjuvant SACT delivered in 2020 compared with 2019 (31% vs. 2%). This is mainly explained by the fact that immunotherapy was approved in the UK in December 2018. Hence, an inevitable and exponential increase in non‐palliative SACT for melanoma was seen, unrelated to the pandemic. Most skin cancers are initially treated with surgery. 5 However, a reduction was seen in skin cancer surgeries during the first wave of the pandemic in the UK. 6 , 7 Therefore, the increase in adjuvant SACT administered may be explained by the clinical decision to start systemic treatment while waiting for final surgical treatment. Lastly, a significant decrease (30%) was observed in the number of palliative SACT. This may have been to avoid patients in a more vulnerable state with higher risk of developing severe COVID‐19 disease from getting infected. Moreover, the redeployment of healthcare professionals and reported shortages of specific resources used in palliative care medicine (i.e. PPE, drugs) may also have compromised the provision of palliative care. 8
Our findings suggest that the COVID‐19 minimal pathways implemented here are safe for patients who require SACT for skin cancers. Although cancer patients have been previously identified as high risk for severe COVID‐19 disease, the implementation of our minimal pathways were designed to keep patients and healthcare professionals from contracting the virus while continuing with life‐saving cancer care.
FUNDING INFORMATION
This work received no external funding.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Maria Jose Monroy‐Iglesias and Hajer Hadi contributed equally to this work as co‐first authors.
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons.
REFERENCES
- 1. Bhargava S, Negbenebor N, Sadoughifar R, Ahmad S, Kroumpouzos G. Global impact on dermatology practice due to the COVID‐19 pandemic. Clin Dermatol. 2021;39(3):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldust M, Agarwal K, Podder I, Navarini AA. Skin cancer and COVID‐19. Dermatol Ther. 2020;33(6):e14050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elmas OF, Demirbas A, Duzayak S, Atasoy M, Tursen U, Lotti T. Melanoma and COVID‐19: A narrative review focused on treatment. Dermatol Ther. 2020;33(6):e14101. [DOI] [PubMed] [Google Scholar]
- 4. Nahm SH, Rembielak A, Peach H, Lorigan PC, Contributing C. Consensus guidelines for the management of melanoma during the COVID‐19 pandemic: surgery, systemic anti‐cancer therapy, radiotherapy and follow‐up. Clin Oncol (R Coll Radiol). 2021;33(1):e54–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sacco AG, Daniels GA. Adjuvant and Neoadjuvant Treatment of Skin Cancer. Facial Plast Surg Clin North Am. 2019;27(1):139–50. [DOI] [PubMed] [Google Scholar]
- 6. Monroy‐Iglesias MJ, Tagliabue M, Dickinson H, Roberts G, De Berardinis R, Russell B, et al. Continuity of cancer care: the surgical experience of two large cancer hubs in London and Milan. Cancers (Basel). 2021;13(7), 1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nolan GS, Dunne JA, Kiely AL, Pritchard Jones RO, Gardiner M, Collaborative RS, et al. The effect of the COVID‐19 pandemic on skin cancer surgery in the United Kingdom: a national, multi‐centre, prospective cohort study and survey of Plastic Surgeons. Br J Surg. 2020;107(12):e598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schoenmaekers J, Hendriks LEL, van den Beuken‐van Everdingen MHJ. Palliative care for cancer patients during the COVID‐19 pandemic, with special focus on lung cancer. Front Oncol. 2020;10:1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reasons.


