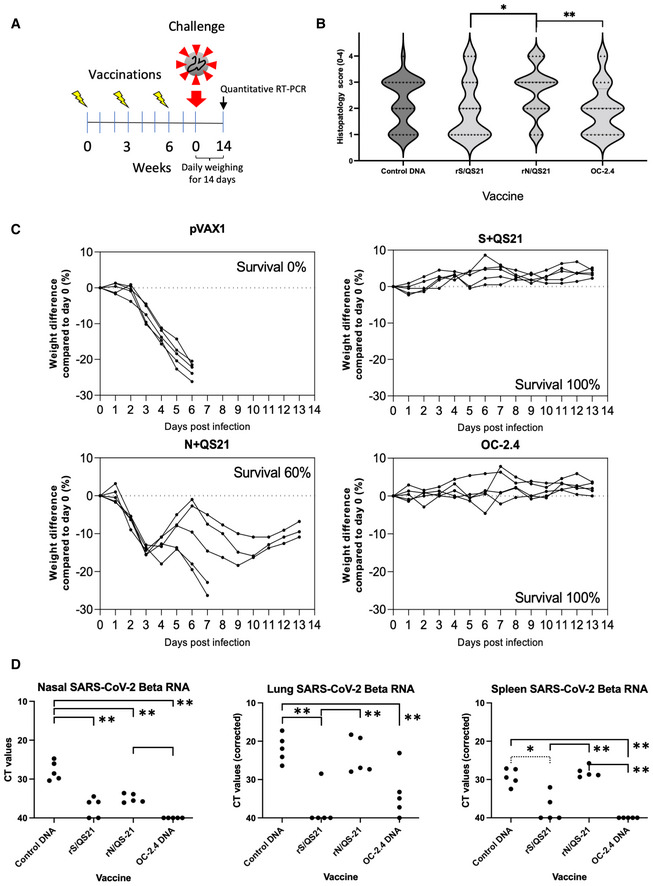
Figure 3. The universal SARS‐CoV‐2 vaccine protects against K18 mice against lethal challenge with SARS‐CoV‐2 Beta variant.
-
AThe experimental design of evaluation of different vaccine strategies in the K18‐hACE2 mice transgenic for the human ACE2 receptor.
-
B, CThree doses of respective vaccine fully or partially protected the mice against severe disease as determined by histological scoring of bronchial and alveolar lung tissues (B), percent weight loss (C).
-
DThree doses of respective vaccine also protect against viral replication in the nose, lungs, and spleen. Values have been given as cycle times (CT), where lower values indicate a higher viral load. Statistical comparisons in the graph are shown with lines with one asterisk indicating P < 0.05 and two asterisks P < 0.01 (Mann–Whitney U‐test, GraphPad Prism).
Data information: The histological scoring (B) was done by an independent pathologist unaware of the experimental groups. The data has been given as the individual histopathological score for each determination in each mouse ranging from 0 (none) to 4 (marked/severe) tissue damage. The weights were given as daily determinations of each mouse during the 14 study period (C). The levels of SARS‐CoV‐2 RNA in nasal washing has been given as the mean cycle time value from a duplicate determination of each sample. The levels of SARS‐CoV‐2 RNA in lung and spleen tissues has been given as the mean cycle time (CT) value from a duplicate determination. Each value was normalized by multiplying individual CT values with the following factor: the mean of all actin CT values divided by the sample actin CT value.
Source data are available online for this figure.
