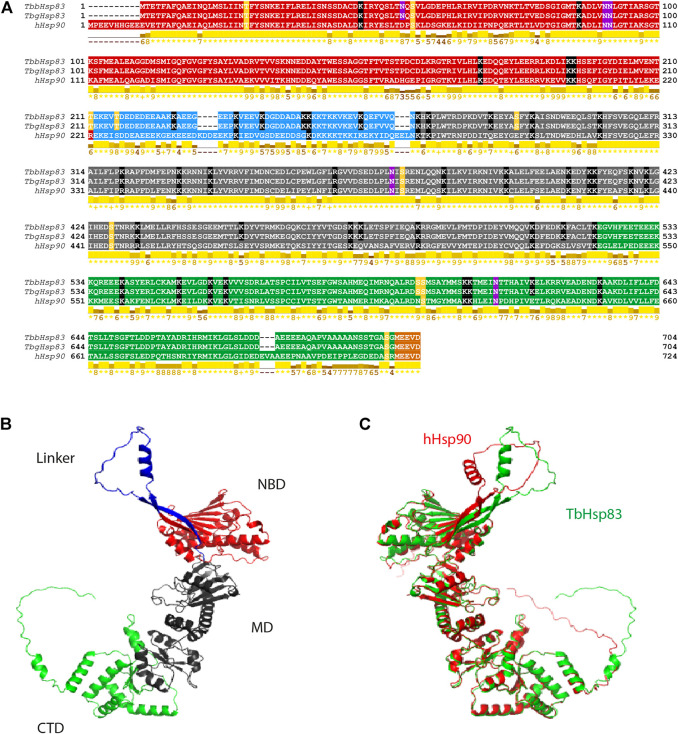
FIGURE 2.
Sequence alignment and 3D structural analysis of TbHSP90. (A) Multiple sequence alignment of hHSP90, TbbHSP83, and TbgHSP83. The NTD is highlighted in red, the charged linker domain in blue, the MD in gray, the CTD in green, and the MEEVD motif in orange. The PTMs are highlighted: yellow–phosphorylation, black—acetylation, and pink—N-glycosylation. Conserved residues involved in ATP interaction are highlighted in brown. Conservation based on physico-chemical properties is shown as a numerical index at the bottom of the alignment: “*” denotes score of 11 where amino acid residues are identical; “+” denotes score of 10 and indicates all properties are conserved. (B) Predicted 3D structure of the TbHSP83 monomer and (C) superimposed 3D structures of TbHSP83 (green) and hHSP90 (red).