Figure 3.
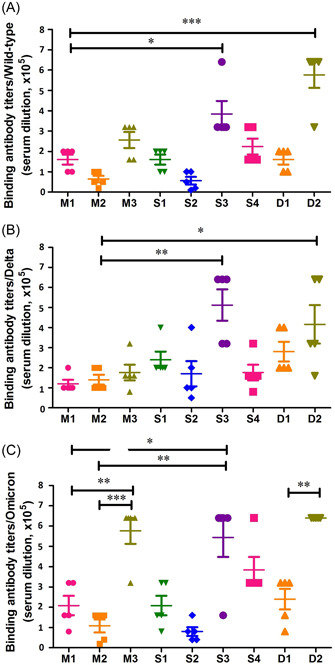
Antispike trimer‐binding antibody titers in monovalent, combination, and sequential vaccination regimens. 96‐well enzyme‐linked immunosorbent assay (ELISA) plates were coated with 2 μg/ml of spike trimer from wild type (A), Delta (B), and Omicron (C) severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) variants. A series dilution of the mouse immune sera from three groups were prepared and incubated with the spike‐coated wells, respectively. Endpoint titers were defined as the highest dilution of serum to give an OD450 > 2.1‐fold of the negative control value. The difference of binding antibody titer was tested for statistical significance with a unpaired t test in the GraphPad Prism 8. Differences were considered statistically significant at *p < 0.05, **p < 0.01, or ***p < 0.001.
