Abstract
The aim of this study is to investigate the relationship between the model for end‐stage liver disease (MELD) score and disease progression and mortality in COVID‐19 patients. The files of 4213 patients over the age of 18 who were hospitalized with the diagnosis of COVID‐19 between March 20, 2020 and May 1, 2021 were retrospectively scanned. Sociodemographic characteristics, chronic diseases, hemogram and biochemical parameters at the time they were diagnosed with COVID‐19 of the patients, duration of hospitalization, duration of intensive care unit (ICU), duration of intubation, in‐hospital mortality from COVID‐19 and outside‐hospital mortality for another reason (within the last 1 year) and recurrent hospitalization (within the last 1 year) were recorded. The MELD scores of the patients were calculated. Two groups were formed as MELD score < 10 and MELD score ≥ 10. The rate of ICU, in‐hospital mortality from COVID‐19 and outside‐hospital mortality from other causes, intubation rate, and recurrent hospitalization were significantly higher in the MELD ≥ 10 group. The duration of ICU, hospitalization, intubation were significantly higher in the MELD ≥ 10 group (p < 0.001). As a result of Univariate and Multivariate analysis, MELD score was found to be the independent predictors of ICU, in‐hospital mortality, intubation, and recurrent hospitalization (p < 0.001). MELD score 18.5 predicted ICU with 99% sensitivity and 100% specificity (area under curve [AUC]: 0.740, 95% confidence interval [CI]: 0.717–0.763, p < 0.001) also MELD score 18.5 predicted in‐hospital mortality with 99% sensitivity and 100% specificity (AUC: 0.797, 95% CI: 0.775–0.818, p < 0.001). The MELD score was found to be the independent predictors of in‐hospital mortality, ICU admission, and intubation in COVID‐19 patients.
Keywords: COVID‐19, intensive care unit admission, in‐hospital mortality
1. INTRODUCTION
In the literature, different results have been reported in various studies concerning hospitalization, intensive care unit (ICU), and mortality in COVID‐19 patients. In one study, the rate of follow‐up in the ICU was reported as 26%, in‐hospital mortality as 4.3%. 1 In another study, it was reported that in‐hospital mortality was 28% and 97% in patients hospitalized in the ICU. 2
Child–Pugh classification, United Network of Organ Sharing classification, and model for end‐stage liver disease (MELD) scoring are quite reliable classifications that are frequently used in determining the survival time of Child–Pugh and MELD patients with cirrhosis. Initially, it has been used to evaluate the prognosis in cases undergoing transjugular intrahepatic portosystemic shunt. 3 Apart from liver patients, it is also employed as a prognostic marker in heart failure patients. 4 , 5 İdin et al. 6 found an independent risk factor that predicts mortality within 30 days in patients with acute pulmonary embolism. MELD‐XI, a modified version of MELD that does not include the international normalized ratio (INR) in patients using anticoagulants, is a useful score used to predict outcomes in various cardiovascular diseases and cardiovascular interventions. Similarly, the MELD‐albumin score (a modified form created by replacing albumin with INR) is an important marker in demonstrating the clinical results used after heart transplantation and heart valve interventions. 6
The aim of this study is to evaluate the relationship between MELD score and disease progression and mortality in COVID‐19 patients who are admitted to a hospital for the treatment of their condition.
2. MATERIALS AND METHODS
It was approved by the Ordu University Clinical Research Ethics Committee. The files of 4213 patients over the age of 18 who were hospitalized with the diagnosis of COVID‐19 (diagnosed by nasopharyngeal swab using the reverse‐transcription polymerase chain reaction 7 ) at the Ministry of Health Ordu University Training and Research Hospital and Erzurum Regional Training and Research Hospital between March 20, 2020 and May 1, 2021 were retrospectively scanned. Patients with chronic liver disease and those using the oral anticoagulant drugs were excluded from the study. Sociodemographic characteristics, chronic diseases, hemogram, and biochemical parameters at the time they were diagnosed with COVID‐19 of the patients, duration of hospitalization, duration of ICU, duration of intubation, exitus in‐hospital for COVID‐19, and exitus outside‐hospital from another reason were recorded. MELD scores of the patients were calculated. The MELD score was calculated according to the formula [0.957 × loge (creatinine) + 0.378 × loge (bilirubin) + 1.12 × loge (INR) + 0.643] × 10). 3 Two groups were formed as a low‐score group (MELD < 10) and high‐score group (MELD score ≥ 10). 7 Blood biochemistry was studied on Beckman Coulter AU 5800 device. Hemogram parameters were studied on Sysmex XN 9000 device.
2.1. Statistical analysis
The data were tested for normality with the Kolmogorov‐Smirnov test and for homogeneity of variance with the Levene test. Variables that met the assumptions were compared with the Student T‐test, and those that did not meet the assumptions were compared with the Mann–Whitney U test and Welch's t‐test. Categorical data were compared using the χ 2 test. Numerical variables were expressed as mean ± SD and median, and categorical variables as percentages. Univariate analyses were used to determine the effects of parameters and MELD score on in‐hospital mortality, admission to ICU, intubation, out‐of‐hospital mortality from other causes, and recurrent hospitalization. Variables with p < 0.05 were included in the Cox regression model. The parameters were stated to be most related to the adverse event in COVID‐19 in the literature (fibrinogen, troponin, ferritin, d‐dimer, C‐reactive protein [CRP], and age) associated with COVID‐19) were taken. A receiver operating characteristic curve analysis was performed to determine the MELD cutoff value for in‐hospital mortality and ICU admission. SPSS 25.0 Statistical Package Program for Windows (SPSS Inc.) was used for all statistical analyzes.
3. RESULTS
When low‐score (MELD < 10) and high‐score (MELD ≥ 10) patient groups were compared in terms of gender and chronic diseases; the male gender was higher in the MELD ≥ 10 group. The rates of hypertension, coronary artery disease, heart failure, chronic obstructive pulmonary disease, cerebrovascular disease, hyperlipidemia, and chronic kidney disease were found to be higher in the MELD ≥ 10 groups (p < 0.001) (Table 1). When groups with MELD < 10 and MELD ≥ 10 were compared, mean age, white blood cell, fasting blood glucose, aspartate aminotransferase, alanine aminotransferase, gamma‐glutamyl transferase, alkaline phosphatase, lactate dehydrogenase (LDH), total bilirubin, creatinine, blood urea nitrogen, ferritin, CRP, sedimentation, procalcitonin, troponin, and INR were higher, hemoglobin level and platelet level were lower (Table 1).
TABLE 1.
Comparison of groups in terms of age, gender, chronic diseases, and laboratory parameters
| Low‐score group MELD < 10 | High‐score group | p | |
|---|---|---|---|
| N= 3988 | N= 225 | ||
| Age | 61.62 ± 15.9 | 69.97 ± 12.79 | <0.001 |
| Gender (%) | |||
| Male | 48.5 | 68.4 | <0.001 |
| Female | 51.5 | 31.6 | |
| Diabetes mellitus (%) | 27.6 | 26.7 | 0.902 |
| Hypertension (%) | 49.3 | 71.6 | <0.001 |
| Coronary artery disease (%) | 20.9 | 38.7 | <0.001 |
| Heart failure (%) | 4.4 | 16 | <0.001 |
| COPD (%) | 12.6 | 23.6 | <0.001 |
| Cerebrovascular disease (%) | 1.9 | 3.6 | 0.087 |
| Hyperlipidemia (%) | 12.1 | 10.7 | 0.598 |
| Chronic kidney disease (%) | 1.7 | 20 | <0.001 |
| Hemoglobin, g/dl | 13.26 ± 1.81 | 12.7 ± 2.90 | <0.001 |
| White blood cell, 103 µl | 7.83 (2.17–93.65) | 11.44 (3.07–97.17) | <0.001 |
| Platelet, 103 µl | 245.32 (46.9–960) | 198.4 (50–682.5) | <0.001 |
| Fasting blood glucose, mg/dl | 153.96 (60–660) | 166.9 (64–609) | 0,006 |
| Aspartate aminotransferase, IU/L | 44.27 (4–2328) | 116.06 (95–1122) | <0.001 |
| Alanine aminotransferase, IU/L | 43.65 (4–1431) | 91 (8–1094) | <0.001 |
| Gamma‐glutamyl transferase | 58.48 (7–1335) | 99.9 (11.73–1731) | <0.001 |
| Alkaline phosphatase | 91.4 (13–977) | 111.9 (36–752) | <0.001 |
| Lactate degidrogenase | 326.9 (101–1552) | 487 (143–2460) | <0.001 |
| Total bilirubin, mg/dl | 0.63 (0.16–2.7) | 1.75 (0.22–24.8) | <0.001 |
| Creatinine, mg/dl | 1.03 (0.31–9.54) | 2.88 (0.31–10.67) | <0.001 |
| Blood urea nitrogen, mg/dl | 24.23 (5–158.6) | 48.10 (8–150) | <0.001 |
| Ferritin | 395.8 (2.6–5995) | 822.9 (21.4–909) | <0.001 |
| d‐dimer | 3205.25 ± 48775.40 | 7107.43 ± 41067.34 | 0.244 |
| CRP, mg/dl | 54.91 (5–1477) | 112.23 (5–1302) | <0.001 |
| Sedimentation | 28.50 (1–140) | 29.56 (2–91) | <0.001 |
| Fibrinogen | 459.06 (63–1096) | 447 (81–830) | <0.001 |
| Procalcitonin | 0.705 (0.007–90.45) | 16.42 (0.01–241.49) | <0.001 |
| Troponin | 311.98 (0.000–25 000) | 1282.13 (0.002–25 000) | <0.001 |
| INR | 1.36 (0.5–6.97) | 1.66 (0.85–13.08) | <0.001 |
| MELD | 4.12 ± 2.55 | 12.27 ± 2.35 | <0.001 |
Abbreviations: COPD, chronic obstructive pulmonary disease;CRP, C‐reactive protein; INR, international normalized ratio; MELD, model for end‐stage liver disease.
When the MELD < 10 and MELD ≥ 10 patient groups were compared duration of hospitalization, the duration of ICU, the duration of intubation, recurrent hospitalization, ICU rate, intubation rate, in‐hospital mortality from COVID‐19, and outside‐hospital mortality for another reason were significantly higher in the MELD ≥ 10 groups than in the MELD < 10 groups (p < 0.001; Table 2).
TABLE 2.
Comparison of groups in terms of recurrent hospitalization, i̇ntensive care unit, mortality, and intubation
| MELD < 10 | MELD ≥ 10 | p‐value | |
|---|---|---|---|
| n = 3988 | n = 225 | ||
| Recurrent hospitalization, n (%) | |||
| No | 3585 (91.1) | 184 (83.3) | <0.001 |
| There is | 352 (8.9) | 37 (16.7) | |
| Intensive care unit, n (%) | |||
| No | 3454 (88.2) | 113 (52.1) | |
| There is | 461 (11.8) | 104 (47.9) | <0.001 |
| Mortality, n (%) | |||
| No | 3533 (88.6) | 109 (48.4) | |
| In‐hospital mortality from COVID‐19 | 383 (9.6) | 110 (48.9) | <0.001 |
| Outside‐hospital mortality from another reason | 72 (1.8) | 6 (2.7) | |
| Intubation rate, n (%) | 280 (7) | 92 (40.9) | <0.001 |
| Duration of hospitalization | 9.30 ± 7.17 | 11.55 ± 11.90 | <0.001 |
| Duration of intensive care unit | 1.29 ± 4.86 | 4.35 ± 10.33 | <0.001 |
| Duration of intubation | 0.59 ± 3.4 | 2.44 ± 7.32 | <0.001 |
Abbreviation: MELD, model for end‐stage liver disease.
As a result of multiple Cox regression analysis, MELD score found to be an independent predictor of in‐hospital mortality (hazard ratio [HR]: 1.063, 95% confidence interval [CI]: 1.037–1.091, p < 0.001), an independent predictor of ICU admission (HR: 1.163, 95% Cl: 1.133–1.194, p < 0.001), and an independent predictor of presence of intubation (HR: 1.043, 95% CI: 1.009–1.078, p = 0.014; Table 3).
TABLE 3.
Identified independent predictors in‐hospital and long‐term mortality and progression in COVID‐19 patients using univariable and multivariable regression analyses
| Univariate analysis HR | Multivariate analysis HR | |||
|---|---|---|---|---|
| (95% CI) | p‐value | (95% CI) | p‐value | |
| In‐hospital mortality | ||||
| MELD | 1.185 (1.142–1.231) | <0.001 | 1.063 (1.037–1.091) | <0.001 |
| Fibrinogen | 0.999 (0.998–1.000) | 0.036 | 0.999 (0.998–1.000) | 0.012 |
| Troponin | 1.000 (1.000–1.000) | <0.001 | 1.000 (1.000–1.000) | 0.692 |
| Ferritin | 1.001 (1.001–1.001) | <0.001 | 1.000 (1.000–1.000) | <0.001 |
| d‐dimer | 1.000 (1.000–1.000) | 0.029 | 1.000 (1.000–1.000) | 0.704 |
| CRP | 1.003 (1.002–1.005) | <0.001 | 1.001 (1.000–1.002) | 0.077 |
| age | 1.028 (1.019–1.037) | <0.001 | 1.002 (0.994–1.009) | 0.662 |
| Admission intensive care unit | ||||
| MELD | 1.269 (1.216–1.323) | <0.001 | 1.163 (1.133–1.194) | <0.001 |
| Fibrinogen | 1.000 (0.999–1.001) | 0.805 | – | |
| Troponin | 1.000 (1.000–1.000) | <0.001 | 1.000 (1.000–1.000) | <0.001 |
| Ferritin | 1.001 (1.001–1.002) | <0.001 | 1.000 (1.000–1.000) | <0.001 |
| d‐dimer | 1.000 (1.000–1.000) | 0.010 | 1.000 (1.000–1.000) | 0.008 |
| CRP | 1.003 (1.002–1.005) | <0.001 | 1.002 (1.001–1.002) | <0.001 |
| age | 1.074 (1.061–1.087) | <0.001 | 1.050 (1.040–1.060) | <0.001 |
| Intubation | ||||
| MELD | 1.242 (1.189–1.269) | <0.001 | 1.043 (1.009–1.078) | 0.014 |
| Fibrinogen | 1.000 (1.000–1.002) | 0.188 | – | |
| Troponin | 1.000 (1.000–1.000) | <0.001 | 1.000 (1.000–1.000) | 0.767 |
| Ferritin | 1.001 (1.001–1.002) | <0.001 | 1.000 (1.000–1.000) | 0.015 |
| d‐dimer | 1.000 (1.000–1.000) | 0.006 | 1.000 (1.000–1.000) | 0.746 |
| CRP | 1.003 (1.001–1.004) | <0.001 | 1.001 (1.000–1.002) | 0.065 |
| age | 1.054 (1.042–1.067) | <0.001 | 1.003 (0.993–1.013) | 0.543 |
| Exitus outside‐hospital from another reason a | ||||
| MELD | 1.075 (0.992–1.169) | 0.079 | – | |
| Fibrinogen | 0.999 (0.997–1.001) | 0.569 | – | |
| Troponin | 1.000 (0.999–1.000) | 0.189 | – | |
| Ferritin | 1.000 (1.000–1.001) | 0.530 | – | |
| d‐dimer | 1.000 (1.000–1.000) | 0.959 | – | |
| CRP | 1.000 (0.998–1.003) | 0.964 | – | |
| Age | 1.057 (1.034–1.080) | <0.001 | – | |
| Recurrent hospitalization | ||||
| MELD | 1.009 (0.977–1.041) | 0.591 | – | |
| Fibrinogen | 0.999 (0.998–1.000) | 0.111 | – | |
| Troponin | 1.000 (1.000–1.000) | 0.206 | – | |
| Ferritin | 0.999 (0.999–0.999) | <0.001 | 0.999 (0.999–0.999) | <0.001 |
| d‐dimer | 1.000 (1.000–1.000) | 0.001 | 1.000 (1.000–1.000) | 0.011 |
| CRP | 0.932 (0.755–1.152) | 0.516 | – | |
| Age | 1.002 (0.995–1.010) | 0.519 | – | |
Abbreviations: CI, confidence interval; CRP, C‐reactive protein; HR, hazard ratio; MELD, model for end‐stage liver disease.
Since MELD was not significant in univariate analysis, multivariate analysis was not performed.
MELD score 18.5 predicted ICU with 99% sensitivity and 100% specificity (area under curve [AUC]: 0.740, 95% CI 0.717–0.763, p < 0.001; Figure 1; Table 4). MELD score 18.5 predicted in‐hospital mortality with 99% sensitivity and 100% specificity (AUC: 0.797, 95% CI: 0.775–0.818, p < 0.001; Figure 2; Table 4).
FIGURE 1.
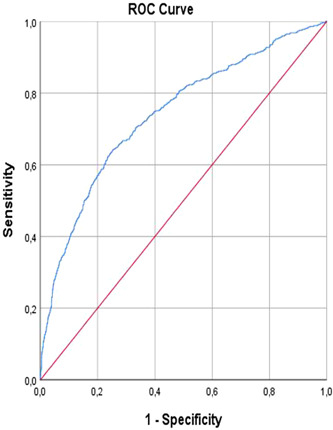
Intensive care unit. ROC, receiver operating characteristic.
TABLE 4.
ROC curve analysis
| AUC | Standard error | p‐value | 95% CI | Sensitivity | Specificity | Cutoff | |
|---|---|---|---|---|---|---|---|
| Exitus in‐hospital (n: 493) | 0.797 | 0.011 | <0.001 | 0.775–0.818 | %99 | %100 | 18.59 |
| Intensive care unit (n: 565) | 0.740 | 0.012 | <0.001 | 0.717–0.763 | %99 | %100 | 18.5 |
Note: p < 0.05.
Abbreviations: AUC, area under curve; CI, confidence interval; ROC, receiver operating characteristic.
FIGURE 2.
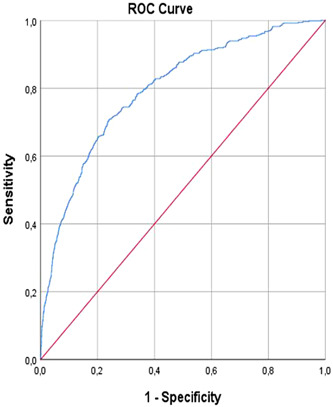
In‐hospital mortality. ROC, receiver operating characteristic.
4. DISCUSSION
In this study, the rates of ICU and intubation, in‐hospital mortality from COVID‐19 and outside‐hospital mortality for another reason, and recurrent hospitalization were significantly higher in the MELD ≥ 10 group (p < 0.001), and the MELD score was found to be an independent predictor of ICU, intubation, and in‐hospital mortality (p < 0.001). An ICU and in‐hospital mortality rate were predicted by a MELD score of 18.5 with 99% sensitivity and 100% specificity (p = 0.001).
As it is known, the rates of admission to the ICU and mortality are high in COVID‐19. As reported in the literature, the case‐fatality rate for COVID‐19 varies markedly with age, ranging from 0.3 deaths per 1000 cases in patients 5–17 years of age to 304.9 deaths per 1000 cases in patients aged 85 and older in the USA. The case fatality rate in patients hospitalized in the ICU is up to 40%. 8 According to the results of 24 observational studies involving 10 150 Covid‐19 (+) patients from centers in Asia, Europe, and North America, the mortality rate of patients treated in the ICU was 41.6%. 9 Approximately, 5% of COVID‐19 patients and 20% of hospitalized patients need intensive care. 8
As a result of various studies, various markers have been found showing intensive care hospitalization and in‐hospital mortality. It was found in studies that d‐dimer, cardiac troponin, CRP, creatinine, alanine transaminase, decreased levels of albumin levels, changes in sodium level, LDH, interleukin‐6, and tumor necrosis factor‐α predicted mortality and disease severity. 10 , 11 , 12 , 13 , 14 Biochemical parameters were examined to predict disease severity and mortality, as well as various scores, were examined in terms of mortality and disease severity with COVID‐19. It was investigated whether scores such as chest computed tomography score and lung ultrasound score predict mortality and disease severity. 15 , 16
As is known, scores were developed to predict disease progression and mortality. There are many scores used in the clinic. Sequential organ failure assessment score is a score used to predict mortality in sepsis, which shows the best‐known multiorgan damage. 17 Simultaneously, various risk scores, such as the COVID‐GRAM critical illness risk score (COVID‐GRAM), the rapid COVID‐19 severity index (qCSI), and the systemic immune inflammation index have been developed to identify critical illnesses in COVID patients. The qCSI is a bedside scale using only three variables (nasal cannula oxygen flow rate, respiratory rate, and minimum documented pulse oximetry). Age, chest X‐ray abnormality, shortness of breath, hemoptysis and confusion, number of comorbid diseases, cancer history, neutrophil–lymphocyte ratio, LDH, and direct bilirubin levels are used to calculate the COVID‐GRAM risk score. 18 As it is known, COVID is a multisystemic infectious disease that affects all organs in the body. COVID‐19 causes multiorgan damage due to severe inflammation and hypoxia (respiratory distress and intravascular thrombus formation). 11 , 19 When calculating the MELD score, creatinine, bilirubin, and INR are used to figure out the score. Creatinine is the parameter that shows kidney functions, INR, and bilirubin liver functions, and liver and kidney functions are also affected in severe COVID‐19 patients.
The MELD score is a score used to determine the priority of liver transplantation in patients with cirrhosis. 20 At the same time, there are studies showing that the MELD score predicts mortality and ICU admission in various diseases. 4 , 5 Bahirwani et al. 21 found that the MELD score predicted in‐hospital mortality of cirrhosis patients admitted to the ICU within 7 days in their study. Al Abbas et al. 22 elective pancreatoduodenectomies (PDs) and distal pancreatectomies in their study on patients found that the MELD score increased twofold in mortality in the MELD > 11 patient group after PDs. Çiftçi et al. 23 found that the MELD‐XI score predicted in‐hospital mortality in patients with moderate to high‐risk pulmonary embolism.
Stawinski et al. 7 in their study to evaluate the relationship between MELD score and COVID‐19, compared in‐hospital mortality or discharge to hospice care, hospital length of stay, and ICU length of stay between groups with low and high MELD scores. They found a difference only in in‐hospital mortality or discharge to hospice care, but they did not find any difference in terms of hospital length of stay or ICU length of stay. As a result, they stated that it is associated with in‐hospital mortality and not with the length of hospital stay and length of stay in the ICU. 7 Unlike this study, we showed that the MELD score is a strong predictor of both in‐hospital mortality and outside‐hospital long‐term mortality in COVID‐19 patients. Also, it was found to be associated with recurrent hospitalizations in the last year. In addition, we showed that the length of hospital stay, length of stay in the ICU, ICU rate, intubation rate, and duration of intubation were significantly increased in patients with high MELD scores.
5. LIMITATION
Our study has some limitations. The main limitations are those of retrospective design. Another limitation is the use of spot laboratory values obtained at the time of admission rather than serial measurements.
6. CONCLUSION
As a result of studies in people infected with COVID‐19, it has been shown that there is an increased inflammatory response (cytokine storm) and this can affect many systems. In addition to the increased inflammatory response, the presence of hypoxia and septic shock increases the risk of multiorgan involvement. It is known that the addition of liver and kidney dysfunctions in critically ill patients increases mortality. The MELD score was developed to assess risk in patients with liver cirrhosis and is actually a practical score that reflects liver and kidney function (based on total bilirubin, creatinine, and INR). These findings suggest that the MELD score can be used as an inexpensive and practical predictor of short‐ and long‐term mortality and morbidity in patients with COVID‐19.
The rapidly globalizing world (increasing travel rates, increasing urbanization, expanding communal areas, etc.) increases the risk of seeing infectious and multisystemic diseases such as COVID‐19. It is obvious that rapid and early estimation of the possible mortality risk in such rapidly spreading disease states will be beneficial in the approach to patients. Consequently, we believe that practical methods such as the MELD score will be useful in determining the risk of mortality and morbidity.
AUTHOR CONTRIBUTIONS
Ahmet Kaya and Yasemin Kaya: Concept.Oktay Gülcü and Ahmet Kaya: Design. Sedat Bostan: Supervision. Yasemin Kaya and Ahmet Kaya: Resource. Yasemin Kaya, Oktay Gülcü, Emrah Aksakal, Kamuran Kalkan, and Sidar Ş. Aydın: Materials.Yasemin Kaya, Oktay Gülcü, Emrah Aksakal, Kamuran Kalkan, Sidar Ş. Aydın, and Ahmet Kaya: Data collection and/or processing. Yasemin Kaya and Sedat Bostan: Analysis and/or interpretation. Ahmet Kaya and Yasemin Kaya: Literature search and writing. Sedat Bostan: Critical review.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Kaya Y, Gülcü O, Aksakal E, et al. A significant predictor of in‐hospital and long‐term mortality and progression in COVID‐19 patients: The end‐stage liver disease (MELD) score model. J Med Virol 2022;1‐8. 10.1002/jmv.28109
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID‐19. Lancet. 2020;395(10229):1014‐1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akdoğan M, Özçay N, Doğrucan N, et al. Karaciğer Transplantasyon Önceliğini Belirlemede Hangi Model EtkinMeld Skoru? Chıld Skoru? Akademik Gastroenteroloji Dergisi 2008;7(2):73‐76. [Google Scholar]
- 4. Kim MS, Kato TS, Farr M, et al. Hepatic dysfunction in ambulatory patients with heart failure: application of the MELD scoring system for outcome prediction. J Am Coll Cardiol. 2013;61:2253‐2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murata M, Kato TS, Kuwaki K, Yamamoto T, Dohi S, Amano A. Preoperative hepatic dysfunction could predict postoperative mortality and morbidity in patients undergoing cardiac surgery: utilization of the MELD scoring system. Int J Cardiol. 2016;203:682‐689. [DOI] [PubMed] [Google Scholar]
- 6. İdin K, Dereli S, Kaya A, et al. Modified model for end‐stage liver disease score predicts 30‐Day mortality in high‐risk patients with acute pulmonary embolism admitted to intensive care units. Scandınavıan Cardıovasc J. 2021;55:237‐244. 10.1080/14017431.2021.1876912 [DOI] [PubMed] [Google Scholar]
- 7. Stawinski PM, Dziadkowiec KN, Al‐Abbasi B, et al. Model of end‐stage liver disease (MELD) score as a predictor of In‐Hospital mortality in patients with COVID‐19: A novel approach to a classic scoring system. Cureus. 2021;13(5):e15179. 10.7759/cureus.15179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;324(8):782‐793. 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 9. Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID‐19: a systematic review and meta‐analysis of observational studies. Anaesthesia. 2020;75(10):1340‐1349. [DOI] [PubMed] [Google Scholar]
- 10. Zhang L, Yan X, Fan Q, et al. D‐dimer levels on admission to predict in‐hospital mortality in patients with Covid‐19. J Thromb Haemost. 2020;18(6):1324‐1329. 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID‐19 patients: a systematic review and meta‐analysis. J Med Virol. 2020;92(10):1875‐1883. 10.1002/jmv.26050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tzoulis P, Waung Z, Bagkeris E, et al. Dysnatremia is a predictor for morbidity and mortality in hospitalized patients with COVID‐19. J Clin Endocrinol Metab. 2021;106(6):1637‐1648. 10.1210/clinem/dgab107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Henry BM, Aggarwal G, Wong J, et al. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID‐19) severity and mortality: a pooled analysis. Am J Emerg Med. 2020;38(9):1722‐1726. 10.1016/j.ajem.2020.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Del Valle DM, Kim‐Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID‐19 severity and survival. Nat Med. 2020;26(10):1636‐1643. 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Francone M, Iafrate F, Masci GM, et al. Chest CT score in COVID‐19 patients: correlation with disease severity and short‐term prognosis. Eur Radiol. 2020;30(12):6808‐6817. 10.1007/s00330-020-07033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sun Z, Zhang Z, Liu J, et al. Lung ultrasound score as a predictor of mortality in patients with COVID‐19. Front Cardiovasc Med. 2021;8:633539. 10.3389/fcvm.2021.633539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khwannimit B, Bhurayanontachai R, Vattanavanit V. Comparison of the accuracy of three early warning scores with SOFA score for predicting mortality in adult sepsis and septic shock patients admitted to intensive care unit. Heart Lung. 2019;48(3):240‐244. 10.1016/j.hrtlng.2019.02.005 [DOI] [PubMed] [Google Scholar]
- 18. Sevinc C, Demirci R, Timur O. Predicting hospital mortality in COVID‐19 hemodialysis patients with developed scores. Semin Dial. 2021;34(5):347‐359. 10.1111/sdi.13004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38:1‐9. 10.12932/AP-200220-0772 [DOI] [PubMed] [Google Scholar]
- 20. Peng Y, Qi X, Guo X. Child–Pugh versus MELD score for the assessment of prognosis in liver cirrhosis: a systematic review and meta‐analySis of observational studies. Medicine. 2016;95(8):e2877. 10.1097/MD.0000000000002877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bahirwani R, Ghabril M, Forde KA, et al. Factors that predict short‐term intensive care unit mortality in patients with cirrhosisclin gastroenterol hepatol. Clin Gastroenterol Hepatol. 2013;11(9):1194‐1200. 10.1016/j.cgh.2013.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Al Abbas AI, Borrebach JD, Bellon J, Zureikat AH. Does preoperative MELD score predict adverse outcomes following pancreatic resection: an ACS NSQIP analysis. Gastrointest Surg. 2020;24(10):2259‐2268. 10.1007/s11605-019-04380-0 [DOI] [PubMed] [Google Scholar]
- 23. Çiftci O, Çelik ÇO, Uzar G, Küpeli E, Müderrisoğlu İH. MELD‐XI score predicts in‐hospital mortality independent of simplified pulmonary embolism severity index among patients with intermediate‐to‐high risk acute pulmonary thromboembolism. Tuberk Toraks. 2019;67(3):169‐178. 10.5578/tt.68614 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


