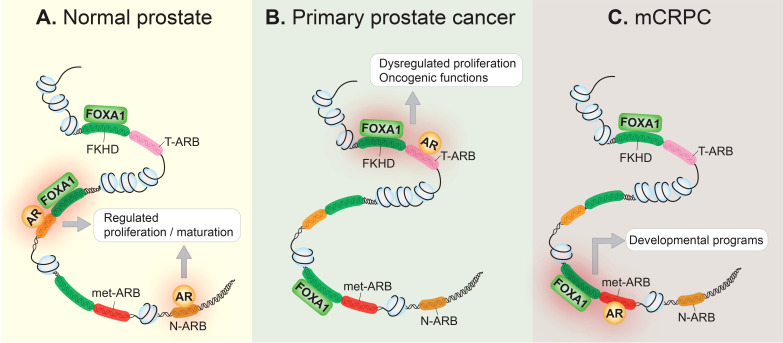
Figure 1.
The AR cistrome is reprogrammed during prostate cancer onset and progression. (A) In the normal prostate epithelium, the AR occupies its normal cistrome, the collective of normal AR binding sites (N-ARB), and controls the AR signaling axis to regulate cellular proliferation, and the maturation of the prostate gland. Pioneer transcription factors, such as FOXA1 which binds to the forkhead (FKHD) binding motif, contribute to AR-regulated transcription. (B) In primary prostate cancer, the AR is redirected to a distinct set of tumor-associated AR binding sites (T-ARB), resulting in aberrant cellular proliferation, transforming the AR into an oncogene. This may be due to genetic or epigenetic disturbances that disrupt the homeostatic relationship between FOXA1 and the AR in normal prostate epithelium. (C) In metastatic castration-resistant prostate cancer (mCRPC), the AR cistrome is further reprogrammed, and AR binds to metastatic AR-binding sites (met-ARB), activating decommissioned developmental programs, seemingly to drive metastasis. FOXA1 binding at these metastatic-associated binding sites precedes the development of mCRPC.