Abstract
Background and aims
Alcohol consumption increased in the early phases of the COVID‐19 pandemic in the United States. Alcohol use disorder (AUD) and risky drinking are linked to harmful health effects. This paper aimed to project future health and cost impacts of shifts in alcohol consumption during the COVID‐19 pandemic.
Design
An individual‐level simulation model of the long‐term drinking patterns for people with life‐time AUD was used to simulate 10 000 individuals and project model outcomes to the estimated 25.9 million current drinkers with life‐time AUD in the United States. The model considered three scenarios: (1) no change (counterfactual for comparison); (2) increased drinking levels persist for 1 year (‘increase‐1’) and (3) increased drinking levels persist for 5 years (‘increase‐5’).
Setting
United States.
Participants
Current drinkers with life‐time AUD.
Measurements
Life expectancy [life‐years (LYs)], quality‐adjusted life‐years (QALYs), alcohol‐related hospitalizations and associated hospitalization costs and alcohol‐related deaths, during a 5‐year period.
Findings
Short‐term increases in alcohol consumption (increase‐1 scenario) resulted in a loss of 79 000 [95% uncertainty interval (UI]) 26 000–201 000] LYs, a loss of 332 000 (104 000–604 000) QALYs and 295 000 (82 000–501 000) more alcohol‐related hospitalizations, costing an additional $5.4 billion ($1.5–9.3 billion) over 5 years. Hospitalizations for cirrhosis of the liver accounted for approximately $3.0 billion ($0.9–4.8 billion) in hospitalization costs, more than half the increase across all alcohol‐related conditions. Health and cost impacts were more pronounced for older age groups (51+), women and non‐Hispanic black individuals. Increasing the duration of pandemic‐driven increases in alcohol consumption in the increase‐5 scenario resulted in larger impacts.
Conclusions
Simulations show that if the increase in alcohol consumption observed in the United States in the first year of the pandemic continues, alcohol‐related mortality, morbidity and associated costs will increase substantially over the next 5 years.
Keywords: alcohol consumption, alcohol‐related liver disease, alcohol‐related hospitalizations, alcohol use disorder, alcohol‐related morbidity and mortality, COVID‐19, health utility, hospitalization cost, simulation model
INTRODUCTION
The COVID‐19 pandemic and the strategies implemented to mitigate its spread have introduced new life stressors and disrupted daily living for most people since March 2020. Individuals often increase alcohol intake to cope with emotional stress and chronic uncertainty [1]. Experience with previous outbreaks [2], natural disasters [1, 3, 4] and terrorist attacks [5, 6] shows sustained increases in alcohol consumption, prevalence of alcohol use disorders (AUD) and alcohol‐attributable violence and death. However, the scale and duration of the current pandemic is unmatched by those isolated events, and it is urgent to monitor alcohol consumption and related problems during and after the pandemic.
AUD is a chronic relapsing illness characterized by an impaired ability to stop or control alcohol use despite adverse social, occupational or health consequences. It is characterized by high mortality and burden of disease world‐wide [7], mainly due to medical consequences, such as liver cirrhosis or injury [8]. An increase in drinking during the COVID‐19 pandemic will add to the known disease burden associated with alcohol [7].
Two recent systematic reviews covered peer‐reviewed literature on alcohol consumption during the COVID‐19 pandemic published up to November 2020 [9] and up to March 2021 [10]. Roberts et al. [9] included studies from 17 different countries and, despite mixed findings, they concluded that overall there was a trend towards increased alcohol consumption during the COVID‐19 pandemic in several countries. Schmidt et al. [10] included studies from more than 19 countries and concluded that risky pre‐pandemic alcohol use, care‐giving responsibilities, stress, depression, anxiety and current treatment for a mental disorder were found to be associated with increased alcohol use. In both reviews studies were not directly compared, nor was a rigorous meta‐analysis conducted due to wide variation in measures and methods between the studies. While changes in alcohol consumption were mixed in many countries, both reviews show that most studies in the United Kingdom and United States found an overall increase in alcohol consumption in the general population and/or specific subgroups in the early phase of the pandemic [9, 10]. Two studies published after those systematic reviews found increases in the number of drinking days, with frequency of drinking peaking in early May and remaining at increased levels through mid‐July in the United States [11, 12]. An additional longitudinal US study, conducted by our team, found sustained increases in average alcohol consumption up to November 2020 [13, 14, 15]. These findings are a concern, as there is an increased risk of AUD‐related consequences as increased consumption persists.
This study uses a previously developed simulation model of the long‐term drinking patterns of people with life‐time AUD [16] to project the alcohol‐related consequences and associated costs of pandemic‐driven increases in consumption overall and for demographic subgroups in the population of current drinkers with a life‐time AUD diagnosis before the COVID‐19 pandemic in the United States. Additionally, we project costs and consequences of pandemic‐driven increases in consumption under scenarios representing sustained changes in consumption. These scenarios help us understand the impacts of observed drinking patterns that might persist beyond the pandemic and inform the need for future interventions.
METHODS
Overview
We used a validated individual‐level simulation model of the long‐term drinking patterns for US adults with life‐time AUD diagnoses [16]. Different model types are better suited for different questions [17]. Individual‐level models (or microsimulation models) simulate one individual at a time and keep track of each simulated individual’s history (e.g. number of hospitalizations or length of time drinking at a specific level) [18].
Individuals in the model transitioned between five mutually exclusive states of alcohol consumption—abstinent, low risk, medium risk, high risk and very high risk—based on the World Health Organization [19] drinking risk levels. Because the population of focus is individuals with life‐time AUD who were drinking immediately before the pandemic, individuals in the model start in one of the four drinking risk levels. Transition probabilities between drinking states varied by age (18–35, 36–50, 51–60, 61–70, 71–80, > 80 years), sex (male or female) and race/ethnicity (American Indian or Alaska Native, Asian or Pacific Islander, non‐Hispanic black, non‐Hispanic white or Hispanic). Individuals also faced morbidity and mortality risks for 28 alcohol‐related conditions that varied by demographic characteristics and drinking risk level. Individuals progressed in the model until they died from alcohol‐related conditions or other causes. Output from the model was used to estimate the impact of increases in alcohol consumption observed during the COVID‐19 pandemic under scenarios representing different durations of increased consumption. Three scenarios were considered: a counterfactual scenario of no change in drinking (‘no change’), and two increased consumption scenarios: increased drinking levels persist for 1 year (‘increase‐1’) and increased drinking levels persist for 5 years (‘increase‐5’). We examined differences in life expectancy [or life‐years (LYs)], quality‐adjusted life‐years (QALYs) [20], alcohol‐related hospitalizations and associated hospitalization costs and alcohol‐related deaths between each increased consumption scenario and the counterfactual (no change) for a follow‐up period of 5 years. We extrapolated model results to the population of US adult drinkers with life‐time AUD. To estimate the size of this population, and because there is no current estimate of the prevalence of life‐time AUD, we multiplied the 2019 prevalence of current (past‐year) AUD for individuals 18 years or older in the United States (14.1 million) from the National Survey on Drug Use and Health (NSDUH) [21] by the proportion of life‐time AUD to current AUD among current drinkers in the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)‐III (1.83) [22, 23], resulting in an estimated 25.9 million current drinkers with life‐time AUD at the beginning of the COVID‐19 pandemic. We conducted supplementary analyses for a life‐time follow‐up horizon, and repeated the 5‐year follow‐up analysis assuming an increase in other‐cause (non‐alcohol related) mortality during the COVID‐19 pandemic (both analyses are presented in Supporting information, Appendices B and D, respectively).
Model parameters
Transition probabilities for drinking states
Short‐term transition probabilities between the five drinking risk states were developed using data from the first and second waves of the NESARC, a nationally representative longitudinal survey of drinking behaviors, conducted between 2001 and 2005 [24, 25]. Long‐term transition probabilities were produced by calibrating the short‐term transition matrices to data from a 16‐year follow‐up study of individuals with life‐time AUD [26, 27], using a simulated annealing algorithm [28]. External validation was conducted using data from NESARC‐III [23, 29], a separate cross‐sectional study conducted in 2012–13 [16]. Additional details are in Supporting information, Appendix A.
Changes to drinking patterns during the COVID‐19 pandemic
Changes in alcohol consumption in the initial phase of the COVID‐19 pandemic were estimated from a nationally representative survey of 993 US adults who were asked to recall their alcohol consumption before (February 2020) and after (April 2020) the pandemic‐related restrictions were put in place in March [13]. Average drinks per day increased significantly by 35% in the survey, with larger increases for women and non‐Hispanic black people. A follow‐up survey with the same sample indicated that the average level of consumption remained elevated to November 2020 [14]. From the first survey data, we estimated average increases in alcohol consumption by sex (i.e. male and female) and race/ethnicity (i.e. non‐Hispanic white, non‐Hispanic black and other race/ethnicity). Because we used demographic‐specific estimates in the model, and because the demographic characteristics vary between the US adult population and the subset of that population with life‐time AUD, the average increase in drinks per day for the model population was 32% [95% confidence interval (CI) = 8–56%] rather than 35% as estimated by the survey. The increase in consumption changes the distribution between drinking states for the period modeled in each scenario (1 or 5 years or life‐time). These increases were incorporated into the model by calibrating each demographic group’s transition probability matrices to produce the observed level of increase in average drinks per day after one model cycle, relative to the counterfactual. We accounted for uncertainty in the estimated changes in consumption by modelling the upper and lower bounds of the 95% CIs for changes in consumption for each demographic group. Additional details on the calibration process are in Supporting information, Appendix A, and changes in alcohol consumption for each demographic group are in Supporting information, Appendix A, Table A1.
Alcohol‐related mortality and morbidity
We selected 28 alcohol‐related conditions based on evidence of causality (Supporting information, Appendix A, Table A2) [30, 31, 32, 33, 34]. To estimate the risk of death for each condition by drinking level, we used sex‐, race/ethnicity‐ and age‐specific deaths for each condition and US population size from the Center for Disease Control and Prevention (CDC) Wonder System WONDER database [35, 36] and risks for individuals with AUD relative to that of the general population from systematic reviews and meta‐analyses [32, 37, 38]. To estimate the risk of hospitalization, we used the number of people with at least one hospitalization event in a year by age, sex, race/ethnicity and condition [39, 40]. See Supporting information, Appendix A for additional details.
Annual hospitalization costs
Annual hospitalization costs for each condition were estimated by age, sex and race/ethnicity using the 2014 health‐care cost and utilization project (HCUP) national inpatient sample (NIS). To obtain costs from HCUP data, we applied a cost‐to‐charge ratio [40] to charges in the NIS at the hospital level. Using costs instead of charges is consistent with a provider perspective on costs. We used the 2014 medical expenditure panel survey (MEPS) [41] to account for the cost of physician services, which is not included in HCUP data. MEPS is the only publicly available data source with estimates of the cost of physician services in the hospital setting. A cost of $463 per day was multiplied by the average length of stay (estimated from the NIS) for each condition by age group. We also used the HCUP national readmissions database (NRD) [39, 40] to account for the fact that some people have multiple hospitalizations for the same condition each year. Accounting for multiple hospitalizations per year increased annual hospitalization costs by 10–20%, on average. Supporting information, Appendix A explains in detail how data from each of these sources were combined to estimate annual hospitalization costs. All costs were inflated to 2020 dollars using the medical care services component of the consumer price index for all urban consumers [42].
Health utility
Health utility values were calculated for each drinking state and alcohol‐related conditions using health utility values and consumption from NESARC‐III [43], as described in Barbosa et al. [44]. Health utility varied by demographics, drinking risk state and alcohol‐attributable conditions (Supporting information, Appendix A, Tables A3 and A4). Utility values were used to compute QALYs. QALYs are years of life adjusted by the quality of those years, with quality measured by health state utilities anchored at 0 for deceased and 1 for perfect health and are used as a standard measure of health that can compare health impacts across different interventions, settings and conditions.
Simulation model
Drinking trajectories and related consequences were simulated for 10 000 individuals over life‐time, and the base‐case results shows model outcomes for the first 5 years of the simulation (life‐time results are in Supporting information, Appendix B). Starting age, sex, race/ethnicity and initial drinking risk state distributions were set to match the population of current drinkers with life‐time AUD, as estimated from weighted NESARC‐III data. To assess the impact of changes in current consumption (i.e. excluding abstainers), all individuals entered the model in one of the four non‐abstinent states (Table 1). Individuals transitioned between the five drinking risk states in annual cycles according to the previously described transition probabilities.
TABLE 1.
Simulation model starting population: descriptive statistics
| Characteristic | NESARC‐III |
|---|---|
| n | 10 000 |
| Average age (SD) | 43.1 (12.4) |
| Sex, n (%) | |
| Male | 6709 (67%) |
| Female | 3291 (33%) |
| Race/ethnicity, n (%) | |
| Non‐Hispanic white | 7535 (75.4%) |
| Non‐Hispanic black | 876 (8.8%) |
| Hispanic | 1052 (10.5%) |
| American Indian/Alaska Native | 379 (3.8%) |
| Asian/Pacific Island | 158 (1.6%) |
| Initial drinking risk state, n (%) | |
| Abstinent | 0 (0.0%) |
| Low risk | 5152 (51.5%) |
| Medium risk | 1226 (12.3%) |
| High risk | 1382 (13.8%) |
| Very high risk | 2240 (22.4%) |
| Average drinks per day (SD) | 4.4 (4.4) |
The distribution of the simulation population was based on the characteristics of adult drinkers with life‐time alcohol use disorder (AUD) from weighted National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)‐III data.
SD, standard deviation.
At each model cycle, individuals could be hospitalized with one or more of the alcohol‐attributable conditions or could die from one of the attributable conditions or from any other cause. Each condition was defined as an acute condition (lasting only one cycle), a chronic condition (lasting an average of 5 years) or a permanent condition (lasting until death), and hospitalization costs and utilities were applied accordingly. Once one or more health conditions occurred, following the minimum health utility estimator [45], the minimum of the applicable utilities between health state and chronic condition utilities was applied. For each simulated individual at each model cycle, the model tracked current drinking risk state, hospitalization for alcohol‐related conditions, hospitalization costs, health utility and, for an individual’s final cycle, the cause of death. QALYs and costs were calculated as the present value of the discounted sum during the 5‐year follow‐up period using a discount rate of 3% [20]. The analyses followed the principles of the consolidated health economic evaluation reporting standards (CHEERS) [46] and other guidelines for reporting simulation models [47, 48, 49]. This analysis has not been pre‐registered on a publicly available platform, and results should be considered exploratory.
RESULTS
The change in average drinks per day in each increased consumption scenario compared with the no change scenario is depicted in Fig. 1. A spike in drinking occurs during the first year of each increased drinking scenario, increasing the average number of drinks per day by approximately one more drink compared to the no change scenario. This initial spike in drinking persists for varying lengths of time in each increased drinking scenario. Average consumption decreases to approximately three drinks per day or fewer by year 10 in all increased drinking scenarios.
FIGURE 1.
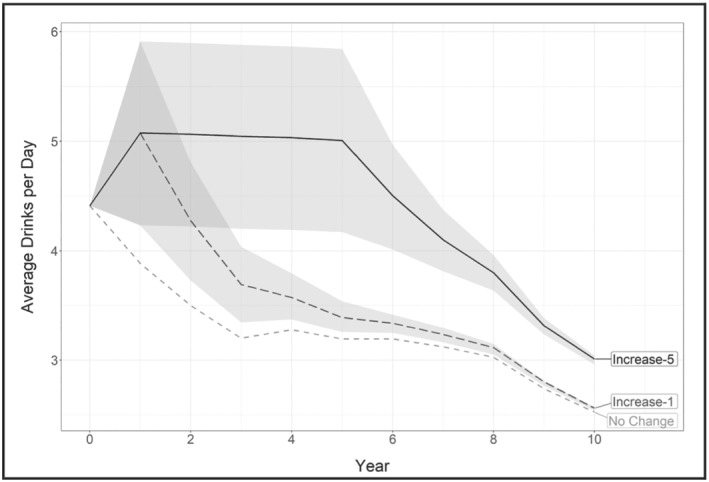
Average drinks per day over the first 10 years by simulation scenario. The shading shows the 95% uncertainty interval for drinks per day (DPD) during the first 10 years of the simulation. Uncertainty diminishes over time once drinking level transition probabilities return to their pre‐COVID levels, because uncertainty intervals in the figure capture the uncertainty related to changes in consumption during the pandemic. With each passing cycle, simulated individuals with alcohol use disorder (AUD) grow closer to the transition probability’s steady state. At the beginning of the simulation (year = 0), the average DPD is 4.4 drinks for all scenarios. In the no change scenario, DPD decreases to 3.2 drinks after 5 years and to 2.5 DPD by 10 years. In the increase‐1 scenario, average DPD increases to 5.1 after year 1, drops to 3.4 DPD by year 5 and to 2.6 DPD by year 10. In the increase‐5 scenario a high drinking level, approximately 5.1 DPD, is sustained for 5 years, dropping to approximately 3 DPD by year 10. Reduced drinking through time results from the long‐term drinking patterns calibrated in the validated model [16], as is also consistent with the general literature that shows that alcohol consumption declines steadily with age [79].
Table 2 shows the average health and cost impacts per person for each scenario, difference in outcomes between each increased drinking scenario and the counterfactual no change scenario and the projected impacts for the population of current drinkers with life‐time AUD. As a result of a shift in the drinking distribution towards higher‐risk drinking states, individuals in the increased drinking scenarios are exposed to a higher risk of alcohol‐related conditions, reflected in lower average LYs and QALYs and higher average hospitalizations and hospitalization costs. Scaling‐up the per‐person results to approximately 25.9 million US adults who are current drinkers with life‐time AUD, short‐term increases in alcohol consumption (increase‐1) resulted in a loss of 79 000 [95% uncertainty interval (UI) = 26 000–201 000] LYs, 332 000 (104 000–604 000) QALYs and 295 000 (82 000–501 000) more alcohol‐related hospitalizations, costing an additional $5.4 billion ($1.5–9.3 billion). Increasing the duration of pandemic‐driven increases in alcohol consumption in the increase‐5 scenario resulted in larger losses in LYs and QALYs and larger increases in hospitalization and associated costs. Analysis using life‐time follow‐up and life‐time increased drinking levels are shown in Supporting information, Appendix B.
TABLE 2.
5‐year follow‐up health and cost impacts for US adults with alcohol use disorder by simulation scenario
| Outcome | No change | Increase‐1 | Increase‐5 | |
|---|---|---|---|---|
| Total health and cost impacts per person | QALYs | 3.32 (SD = 0.42) |
3.30 (SD = 0.42) [UI: 3.29, 3.31] |
3.29 (SD = 0.43) [UI: 3.28, 3.31] |
| LYs | 4.90 (SD = 0.58) |
4.90 (SD = 0.58) [UI: 4.89, 4.90] |
4.89 (SD = 0.59) [UI: 4.89, 4.90] |
|
| Hospitalizations a | 0.23 (SD = 0.50) |
0.24 (SD = 0.51) [UI: 0.23, 0.25] |
0.25 (SD = 0.52) [UI: 0.24, 0.27] |
|
| Hospitalization costs a | $4500 (SD = $10 600) |
$4800 (SD = $10 800) [UI: $4600, $4900] |
$4900 (SD = $11 000) [UI: $4700, $5200] |
|
| Incremental health and cost impacts per person | QALYs | NA |
−0.01 [UI: −0.02, 0.00] |
−0.02 [UI: −0.04, −0.01] |
| LYs |
0.00 [UI: −0.01, 0.00] |
−0.01 [UI: −0.01, 0.00] |
||
| Hospitalizations a |
0.01 [UI: 0.00, 0.02] |
0.02 [UI: 0.01, 0.04] |
||
| Hospitalization costs a |
$200 [UI: $100, $400] |
$400 [UI: $200, $600] |
||
| Incremental health and cost impacts for total US adult AUD population | QALYs | NA |
−332 K [UI: −604 K, −104 K] |
−588 K [UI: −1036 K, −198 K] |
| LYs |
−79 K [UI: −201 K, −26 K] |
−141 K [UI: −269 K, −77 K] |
||
| Hospitalizations a |
295 K [UI: 82 K, 501 K] |
606 K [UI: 276 K, 945 K] |
||
| Hospitalization costs a |
$5.4 B [UI: $1.5 B, $9.3 B] |
$10.4 B [UI: $4.6 B, $16.5 B] |
The first panel of the table presents average QALYs, LYs, hospitalizations and hospitalization costs for each scenario over 5 years. The standard deviation in parentheses represents variation among individuals in the model, and the bracketed uncertainty interval shows the range of the mean assuming a small increase in drinking (7.7%) and a large increase in drinking (55.7%). The second panel shows the difference in average outcomes for each increased drinking scenario compared to the no change scenario for the estimated increase in drinking (31.7%) and then in brackets over the range of assumed increases in drinking (7.7 and 55.7%). The third panel extrapolates the second panel to the population of US adult drinkers with life‐time alcohol use disorder (AUD), by multiplying the individual‐level incremental difference by the number of US adult drinkers with life‐time AUD (25.9 million people in 2019). Costs are in 2020 US dollars.
LYs, life years; NA, not available; QALYs, quality‐adjusted life years; SD, standard deviation; UI, uncertainty interval.
Hospitalizations and hospitalization costs reflect counts and costs for the 28 alcohol‐related conditions (Supporting information, Appendix Table A2).
To compare the impacts of the increased drinking scenarios across race/ethnicity, sex and age strata, we standardized outcomes within these strata by dividing each increased drinking scenario result by the counterfactual value. Figure 2 shows standardized outcomes for QALYs and hospitalization costs during a 5‐year follow‐up period for each increased drinking scenario and stratum. We see a larger decline in QALYs and a larger increase in hospitalization costs for non‐Hispanic black people and people in the other race/ethnicity group, which includes Hispanic people and those of other races. The standardized outcomes also show a larger effect of the increase in consumption for women than for men. By age group, standardized differences in QALYs and costs are most apparent in the increase‐5 scenario. There is a larger cost increase for people aged 36–50 years, and a slightly larger QALY decrease for people aged 51 years or older. Supporting information, Appendix C shows results for LYs and total hospitalizations for the 5‐year follow‐up period.
FIGURE 2.
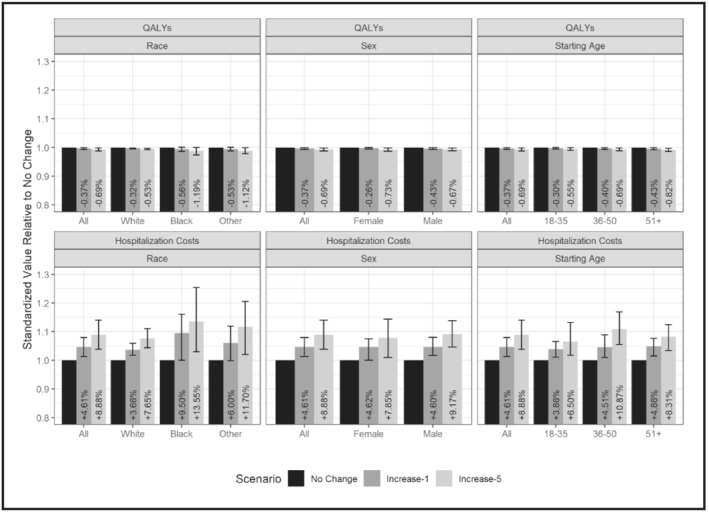
Standardized outcomes per person for each COVID scenario by race, sex and age strata, 5‐year follow‐up. Standardized outcomes computed by dividing each stratum’s value for a particular outcome by the no change value within that scenario. Starting age refers to the age of the individual when entering the model (and the age at which changes in consumption due to COVID took effect). Error bars represent the impact of uncertainty in consumption changes, using the values of the 95% confidence interval of the survey estimates. Percentages represent the difference in the outcome compared to the no change scenario. This figure shows standardized outcomes for quality‐adjusted life‐years (QALYs) and hospitalization costs over a 5‐year follow‐up period for each increased drinking scenario and stratum. We see a larger decline in QALYs and a larger increase in hospitalization costs for non‐Hispanic black people and people in the other race/ethnicity group, which includes Hispanic people and those of races other than black and white. The standardized outcomes also show a larger effect of the increase in consumption for women than for men. By age group, standardized differences in QALYs and costs are most apparent in the increase‐5 scenario. There is a larger cost increase for people aged 36–50 years and a slightly larger QALY decrease for people aged 51 years or older. Supporting information, Appendix Figure B1 shows results for LYs and total hospitalizations for the 5‐year follow‐up period.
Finally, we examined the change in number of hospitalizations and deaths by alcohol‐related condition to identify the conditions most impacted in each scenario (Table 3). For the increase‐1 scenario, hospitalizations for alcohol‐related conditions increased by 295 177 (82 856–502 320) relative to the no change scenario, at an estimated cost of $5.4 billion ($1.5 billion–$9.3 billion). Deaths due to all causes increased by 38 839 (10 358–88 036). For all scenarios, the main driver of increased hospitalizations, their associated costs and deaths was cirrhosis of the liver. In the increase‐1 scenario, hospitalizations and deaths for cirrhosis of the liver increased by approximately 10% [138 821 (41 428–225 268) and 31 072 (10 358–49 197) more cases, respectively] than in the no change scenario (Table 3). These increased hospitalizations for cirrhosis of the liver accounted for approximately $3.0 billion ($0.9 billion–$4.8 billion) additional hospitalization costs, more than half of the increased hospitalizations costs across all alcohol‐related conditions. Pancreatitis and mental and behavioral disorders due to the use of alcohol also accounted for a large share of the incremental hospitalizations. A few conditions showed no increase in deaths or even a decrease in deaths with the increased drinking scenarios (e.g. colon and rectal cancer). This finding is largely due to earlier mortality from cirrhosis and other highly attributable conditions that can result in death at younger ages. Supporting information, Appendix Table B2 shows the life‐time results by condition. The supplementary analysis assessing the impact of increased mortality for all non‐alcohol related causes during the COVID‐19 pandemic suggested that the estimated impact of increased alcohol consumption remained considerable (Supporting information, Appendix D).
TABLE 3.
Estimated 5‐year hospitalization cases, hospitalization costs and deaths by alcohol‐related condition for the US AUD population for no change and incremental impacts under increase‐1 and increase‐5 scenarios
| Diagnosis a | No change totals | Increase‐1 incremental impacts | Increase‐5 incremental impacts1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Costs, $M | Deaths | Cases a | Costs, $M | Deaths | Cases | Costs, $M | Deaths | |
| Cirrhosis of the liver | 1 387 852 | 30 183 | 300 355 | 139 821(+10.1%) | 2987.2(+9.9%) | 31 072(+10.3%) | 261 517(+18.8%) | 5471.8(+18.1%) | 80 267(+26.7%) |
| Pancreatitis | 520 444 | 7665 | 7767 | 46 607(+9.0%) | 699.0(+9.1%) | 0(0.0%) | 93 214(+17.9%) | 1383.7(+18.1%) | 5178(+66.7%) |
| Mental and behavioral disorders due to use of alcohol | 502 319 | 5385 | 20 714 | 31 071(+6.2%) | 327.3(+6.1%) | 0(0.0%) | 95 803(+19.1%) | 981.7(+18.2%) | 0(0.0%) |
| Atrial fibrillation and flutter | 178 660 | 2378 | 0 | 15 536(+8.7%) | 212.1(+8.9%) | 0(0.0%) | 25 893(+14.5%) | 347.2(+14.6%) |
2589 (n/a) |
| Unipolar depressive disorder | 398 748 | 3714 | 0 | 10 357(+2.6%) | 96.0(+2.6%) | 0(0.0%) | 20 714(+5.2%) | 186.3(+5.0%) | 0(0.0%) |
| Epilepsy | 176 071 | 2500 | 5178 | 10 357(+5.9%) | 150.1(+6.0%) | 0(0.0%) | 23 303(+13.2%) | 333.0(+13.3%) | 0(0.0%) |
| Pneumonia | 390 981 | 6574 | 7767 | 7768(+2.0%) | 132.6(+2.0%) | 0(0.0%) | 7768(+2.0%) | 134.5(+2.0%) | 0(0.0%) |
| Unintentional injuries other than transport accidents | 662 855 | 14 493 | 59 553 | 7768(+1.2%) | 204.4(+1.4%) | 5178(+8.7%) | 12 946(+2.0%) | 305.6(+2.1%) | ‐2589(−4.3%) |
| Motor vehicle transport accidents | 406 516 | 14 075 | 18 125 | 5179(+1.3%) | 193.1(+1.4%) | 0(0.0%) | 7768(+1.9%) | 260.4(+1.9%) | ‐2589(−14.3%) |
| Diabetes mellitus | 269 285 | 4217 | 18 125 | 5179(+1.9%) | 65.8(+1.6%) | ‐5178(−28.6%) | 2589(+1.0%) | 31.1(+0.7%) | ‐5178(−28.6%) |
| Intentional self‐harm | 292 588 | 3857 | 119 107 | 5179(+1.8%) | 59.1(+1.5%) | 2589(+2.2%) | 33 661(+11.5%) | 417.7(+10.8%) | 7767(+6.5%) |
| Esophageal varices | 62 143 | 1144 | 0 | 2589(+4.2%) | 39.7(+3.5%) | 0(0.0%) | ‐5179(−8.3%) | −101.5(−8.9%) |
2589 (n/a) |
| Hypertensive heart disease | 7768 | 109 | 12 947 | 2589(+33.3%) | 35.7(+32.6%) | 0(0.0%) | 2589(+33.3%) | 35.7(+32.6%) | 0(0.0%) |
| Hemorrhagic and other non‐ischemic stroke | 51 786 | 2729 | 12 947 | 2589(+5.0%) | 148.3(+5.4%) | −2589(−20.0%) | 5179(+10.0%) | 246.4(+9.0%) | 0(0.0%) |
| Ischemic heart disease | 411 695 | 12 156 | 93 214 | 2589(+0.6%) | 73.5(+0.6%) | 0(0.0%) | 12 946(+3.1%) | 369.2(+3.0%) | −2589(−2.8%) |
| Alzheimer’s and other dementias | 18 125 | 432 | 10 358 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | −1.8(−0.4%) | −2589(−25.0%) |
| Breast cancer | 12 946 | 261 | 10 358 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | 2589(+25.0%) |
| Cardiomyopathy | 0 | 0 | 7767 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | −2589(−33.3%) |
| Colon and rectal cancer | 54 375 | 1633 | 20 714 | 0(0.0%) | 0.0(0.0%) | −5178(−25.0%) | 0(0.0%) | 0.0(0.0%) | −7767(−37.5%) |
| Ischemic stroke | 103 571 | 2013 | 5178 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 7768(+7.5%) | 145.5(+7.2%) | −2589(−50.0%) |
| Larynx cancer | 0 | 0 | 0 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | 0(0.0%) |
| Lip and oral‐cavity cancers | 7768 | 300 | 0 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | 0(0.0%) |
| Liver cancer | 15 536 | 429 | 10 358 | 0(0.0%) | 0.0(0.0%) | 2589(+25.0%) | 0(0.0%) | 0.0(0.0%) | 0(0.0%) |
| Nasopharynx cancer | 0 | 0 | 0 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | 0(0.0%) |
| Esophagus cancer | 10 357 | 404 | 5178 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | −2589(−25.0%) | −94.4(−23.4%) | −2589(−50.0%) |
| Other pharynx cancer | 2589 | 86 | 0 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | 0(0.0%) |
| Pancreatic cancer | 12 946 | 406 | 20 714 | 0(0.0%) | 0.0(0.0%) | 2589(+12.5%) | 0(0.0%) | 0.0(0.0%) | −2589(−12.5%) |
| Tuberculosis | 12 946 | 565 | 2589 | 0(0.0%) | 0.0(0.0%) | 0(0.0%) | 0(0.0%) | 0.0(0.0%) | 2589(+100.0%) |
| All other causes | – | – | 258 927 | – | – | 7767 (+3.0%) | – | – | 2589 (+1.0%) |
| Total | 5 970 869 | 117 708 | 1 027 942 | 295 117 (+4.9%) | 5423 (+4.6%) | 38 839 (+3.8%) | 605 891 (+10.1%) | 10 451 (+8.9%) | 75 089 (+7.3%) |
The table presents hospitalizations, hospitalization costs and deaths due to alcohol‐related conditions in the no change scenario, and then shows the difference in these outcomes (% changes in brackets) for the increase‐1 and increase‐5 scenarios relative to the no change scenario. Deaths are also shown for all other conditions, but hospitalizations and hospitalization costs were not tracked for conditions other than the 28 alcohol‐related conditions. The projected total hospitalizations are the product of the incremental per person results and the estimated population of US adult drinkers with life‐time alcohol use disorder (AUD) (25.9 million people in 2019). Repeating estimates of 2589 is due to the rounding of very small model output values. In the iIncrease‐1 scenario, increased drinking levels persist for 1 year. In the increase‐5 scenario, increased drinking levels persist for 5 years. Costs are in 2020 US dollars.
Note on decreases in deaths: for some conditions (e.g. colon and rectal cancer, pancreatic cancer) there was a decrease in the number of hospitalizations and deaths under the increased drinking scenarios. This was due to earlier mortality, largely related to cirrhosis (see the large increases in cirrhosis deaths) and a few other conditions including intentional self‐harm. Because it takes longer to develop cancers, mortality due to these conditions decreases as cirrhosis and other causes of death increase.
Table rows are sorted in descending order of the number of hospitalization cases in the increase‐1 scenario in the first column of data.
Supporting information, Appendix Table B1 shows similar results for the life‐time follow‐up.
DISCUSSION
Alcohol consumption accounts for a significant health and economic burden in the United States [50, 51, 52, 53], and the increases in consumption observed in the early phases of the pandemic might exacerbate this burden. This study used an individual‐level simulation model of the long‐term drinking patterns for individuals with life‐time AUD [16] to assess the impacts of pandemic‐driven increases in alcohol consumption for current drinkers with life‐time AUD.
Our results show that the increase of about 32% in average drinks per day observed in the first year of the pandemic can substantially increase alcohol‐related mortality, morbidity and associated costs in the next 5 years. When shifting the drinking distribution towards higher‐risk drinking states, individuals are exposed to a higher risk for alcohol‐related conditions, which is reflected in fewer average LYs and QALYs and higher average hospitalizations and hospitalization costs. Increasing the duration of pandemic‐driven increases in alcohol consumption in the increase‐5 scenario, and assuming no further intervention, resulted in larger losses in LYs and QALYs and larger increases in hospitalization and associated costs.
Of the 28 alcohol‐related conditions assessed, cirrhosis of the liver was the largest contributor to an increase in hospitalizations and associated costs. This finding is concerning, because hospitalizations and mortality due to alcohol‐related liver disease have increased substantially over the last decade and our results show that pandemic‐related increases in consumption will increase this burden, even with short‐term increases in consumption. In line with our projections, early in the pandemic there were reports of a spike in hospital admissions for alcohol‐related liver disease during the COVID‐19 pandemic [54, 55], and recent evidence shows that the number of patients awaiting and undergoing liver transplantation for acute alcohol‐associated hepatitis substantially increased during the COVID‐19 pandemic in the United States [56, 57].
Our surveys showed that women had larger relative increases in consumption than men [13, 58]. Other studies have also shown larger relative increases in consumption among women, compared to men, during the COVID‐19 pandemic [9, 10, 59]. Women are more susceptible than men to alcohol‐related health problems, such as liver injury [60]. In the past two decades, alcohol consumption and alcohol‐related emergency department visits, hospitalizations and deaths have increased markedly among women [61]. Our model results show that females had larger increases in hospitalizations and associated costs than men in all scenarios, and larger decreases in LYs and QALY when increases in drinking are sustained, which means that the pandemic is exacerbating these troubling trends. We found a larger impact on costs and health for other groups compared to non‐Hispanic white individuals (more generally). The disproportionate impact of the pandemic on racial and ethnic minorities [62], together with worsening drinking patterns in these subgroups, compounds existing disparities in the consequences of excessive alcohol consumption [63]. Our findings support the need for continued monitoring of alcohol consumption and alcohol‐attributable harms now and beyond the COVID‐19 pandemic, with special attention to minority groups. They also support the need for individual and population‐level interventions to manage AUD and prevent increases in consumption.
Our results underestimate the potential consequences of pandemic‐driven increases in alcohol consumption. Health‐care costs were restricted to hospitalization costs, hence missing outpatient, pharmacy and other health‐care costs. We did not account for decreases in elective health care utilization and disruptions to behavioral health treatment in the early phases of the pandemic [64, 65]. Delays in obtaining care for alcohol‐related conditions can exacerbate the risk of downstream hospitalizations and premature mortality, and the inaccessibility to behavioral health‐care may result in a higher prevalence of AUD. We also did not account for the entire social impacts of excessive consumption, which include impacts on family and friends, productivity, employment and the criminal justice system.
Our individual‐level simulation model tracked alcohol‐related consequences and associated costs for individuals with life‐time AUD and currently drinking at the beginning of the simulation immediately before the COVID‐19 pandemic. Our model does not explicitly account for changes in the incidence of AUD. We scaled‐up individual‐level results to provide an estimate of the impact on the 2019 prevalence of life‐time AUD for drinkers aged 18 years or older in the United States (25.9 million). It is possible that increased alcohol consumption during the COVID‐19 pandemic, combined with pandemic‐related factors, led to an increase in the prevalence of AUD and that we underestimated the impacts of increases in consumption on that prevalence. However, NSDUH, which is the benchmark nationally representative cross‐sectional survey for AUD prevalence in the United States, had disruptions to data collection in 2020 [66], raising questions about the validity of AUD prevalence estimates for 2020, and scaling‐up results with NSDUH 2020 estimates should be performed with caution. In addition, NSDUH 2019 used the 4th edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV) [67] which described two distinct disorders—alcohol abuse and alcohol dependence—with specific criteria for each. NSDUH 2020 used the 5th edition, DSM‐5 [68], which integrates the two DSM‐IV disorders into a single AUD, with mild, moderate and severe subclassifications. It has been shown that AUD prevalence rates are lower with DSM‐IV criteria than DSM‐5 criteria [69]. The 2020 NSDUH current AUD prevalence for individuals aged 18 years or older was 26.3 million. However, because of different diagnostic criteria, the estimate cannot be directly comparable to the 2019 estimate of 14.1 million or other previous years to inform changes in trends. In addition to results scaled‐up to the life‐time AUD population at the beginning of the pandemic, we also provide individual‐level results, which allows another scalar to be easily applied.
One limitation of our individual‐level simulation model is that the longitudinal NESARC data used to model long‐term drinking trajectories was collected between 2001 and 2005. However, these data are unique in the size of the sample, and it is the only longitudinal nationally representative data set with detailed information on drinking patterns for an alcohol‐dependent population in the United States. External validation of long‐term drinking trajectories was conducted with more recent data (NESARC‐III). In addition, the starting model distribution was informed by NESARC‐III because this is the most recent nationally representative survey with detailed information on drinking patterns allowing accurate assignment of individuals with life‐time AUD to the drinking states. NESARC‐III, in combination with NSDUH, was also used to estimate the size of the population of people with life‐time AUD who are currently drinking. Our estimate of the size of that population implicitly assumes that the ratio of current to life‐time AUD has not changed since 2013. There is an urgent need for more recent large‐scale nationally representative longitudinal studies of alcohol and related conditions.
There are four key limitations related to the data used to inform changes in alcohol consumption during the COVID‐19 pandemic. First, the survey used to inform changes in alcohol consumption during the COVID‐19 pandemic was not designed to reach specialized populations, such as those with life‐time AUD, which is the focus of this study. Therefore, we assume that changes in drinking patterns observed in the general US population can be transferred to changes in the population of current drinkers with life‐time AUD. To date, no study has specifically assessed changes in drinking patterns during the COVID‐19 pandemic for the AUD population, which is a considerable gap in the existing literature on COVID impacts. However, several studies that examined pre‐COVID alcohol use or severity as a predictor of changes in consumption found a positive relationship between increases in consumption and preCOVID alcohol use severity [59]. Therefore, our results might be an underestimate of the impacts for the life‐time AUD population if people with a history of AUD have disproportionately larger increases in consumption during the pandemic [62]. To account for this uncertainty, we also modeled the impact of smaller [8 versus 32% (averages across demographic groups, change in consumption in the model varied by race/ethnicity and sex)] and larger (56% versus 32%) changes in alcohol consumption. Secondly, alcohol consumption changes were taken from one single survey with approximately 1000 people. Most studies to date used convenience samples and did not collect detailed alcohol consumption data, mainly simply asking if consumption increased or decreased since the start of the COVID‐19 pandemic. The study we used is the only nationally representative study to date that collected detailed data on alcohol consumption allowing accurate estimation of average consumption per day overall and by sex and race/ethnicity subgroups. There are three other nationally representative studies of adult alcohol consumption in the United States. The study by Pollard and colleagues [70] used the RAND Corporation American life panel and found a 14% increase in frequency of alcohol use in May 2020 compared to 2019, with a 17% increase for women. They also found a 41% increase in days of heavy drinking for women. However, the study only included individuals aged 30–80 years and did not collect detailed alcohol consumption pattern data. Two studies used the Understanding America study (UAS) to examine longitudinal changes in number of drinking days in the past week from March 2020 to June 2020 [11] or 21 July 2020 [12]. Both studies found increases in the number of drinking days, with frequency of drinking peaking in early May and remaining at increased levels through mid‐July [11, 12]. However, the UAS survey did not collect measures of quantity consumed and thus could not examine changes in the total quantity of alcohol consumed per day or other changes in drinking patterns. Individuals might increase drinking frequency yet still reduce average consumption if the amount consumed on each occasion decreases, or they might increase consumption to the point of placing themselves and others at increased risk of harm. As the number of studies on changes in alcohol consumption during the COVID‐19 pandemic grows, there is an increased need to combine results through meta‐analyses. A recent study conducted a systematic review and meta‐analysis of changes in alcohol use during COVID‐19 including 128 studies with data from 58 countries [59]. The study revealed that 23% of participants reported increases in consumption and 23% reported decreases in the early phase of the pandemic. There was significant heterogeneity across studies for both increase and decrease effect sizes, with the country moderator revealing a larger increase in the United States and several European countries. The high variability in methods and alcohol measures across studies challenges a meta‐analysis on drinking patterns, in particular quantity, frequency and individual‐level consumption. Future studies should collect both quantity and frequency of standard drinks information to provide an accurate assessment of drinking patterns so that meta‐analyses of the impact of the COVID‐19 pandemic on level of consumption and magnitude of changes can be conducted. Thirdly, while the survey that informed drinking during the COVID‐19 pandemic was cross‐sectional and prone to recall bias, the use of an anchoring event (the beginning of the pandemic) was designed to increase the accuracy of recalled consumption when February consumption was asked in May 2020 [13]. In addition, a follow‐up survey with the same sample confirmed sustained increases in consumption [13, 14, 15], and the other three longitudinal studies conducted in the United States so far [11, 12, 70], despite not providing detailed information on drinking patterns, also found increases in alcohol consumption during the COVID‐19 pandemic. Furthermore, the meta‐analysis by Acuff et al. (2002) found no difference between cross‐sectional and longitudinal studies in the size of the effect, and recent research in Canada revealed longitudinal patterns of drinking that were substantively consistent with self‐attributions [71]. A fourth limitation is that our estimates of alcohol consumption may underestimate total consumption, which is a well‐known limitation of collecting self‐reported data on substance use using surveys [72, 73]; however, this approach is still considered reliable for measuring alcohol consumption [74].
While we presented our results for a wide interval of increase in alcohol consumption (average between 8 and 56%) it is possible that the increase in consumption for current drinkers with life‐time AUD was even smaller than 8%. Sales data indicate an increase on apparent per‐capita alcohol consumption of 2.9% in 2020 compared to 2019 [75]. While this is a large increase, it is smaller than our lower‐bound estimate and it is possible that the real impact of pandemic‐driven increases falls between the counterfactual scenario (i.e. no increase in drinking) and the lower‐bound increase estimates presented. However, the 2.9% estimate is calculated by dividing gallons of ethanol (pure alcohol) by each state’s resident population aged 14 and older to obtain per‐capita ethanol estimates, whereas the estimate used in our study, and those of other studies of self‐reported alcohol consumption during the COVID‐19 pandemic that reported estimates similar to ours, used individuals of drinking age (older than 21 years). It is possible that those of drinking age increased consumption more than individuals aged between 14 and 20 years [76, 77]. In addition, while sales data and self‐reported consumption are correlated they do not always coincide. It is possible that the totality of the increase in consumption was not captured by sales data due to consumption of existing alcohol stocks in the household, particularly spirits. Individuals may have consumed what they had on hand during the early phases of the pandemic as they attempted to socially distance, and policies limited access to vendors. This may have been followed by replenishment, a notion that is supported by a 7.1% increase in spirits sales between 2019 and 2020 (more than twice as large as the next largest year‐on‐year increase recorded since 1971) [75], and evidence from 13 states that the increase in spirits sales was even higher in 2021 [78].
This study assessed the impact of pandemic‐driven increases in alcohol consumption on the short‐ and long‐term alcohol‐related health consequences and associated costs for adult drinkers with life‐time AUD in the United States. Results were stratified by sex, age and race/ethnicity. We showed that the health and cost impacts of pandemic‐driven increases in alcohol consumption are substantial, disproportionally impact minorities, and if those increases persist, the toll will be larger. Understanding the short‐ and long‐term consequences of increases in consumption during and after the pandemic provides critical information to clinicians, policymakers and public health agencies to more efficiently plan for health services and prepare for future public health emergencies in the United States and abroad.
DECLARATION OF INTERESTS
None.
AUTHOR CONTRIBUTIONS
Carolina Barbosa: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; supervision; validation; visualization. Simon Neuwahl: Formal analysis; methodology. Jurgen Rehm: Conceptualization; data curation; funding acquisition; investigation; methodology. Sameer Imtiaz: Formal analysis; methodology. Gary Zarkin: Conceptualization; funding acquisition; investigation; methodology; supervision. William Dowd: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; resources; software; validation.
Supporting information
Appendix A. Model Parameter Details and Methods
Appendix B. Additional Model Results, Life‐time Follow‐Up
Appendix C. Additional Model Results, 5‐year Follow‐Up
Appendix D. Assessing the Impact of Increased Mortality for all Non‐Alcohol‐Related Causes during the COVID‐19 Pandemic
ACKNOWLEDGEMENTS
Financial support for this study was provided in part by the National Institute on Alcohol Abuse and Alcoholism under Award Number R01AA024423. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing and publishing the report. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Barbosa C, Dowd WN, Neuwahl SJ, Rehm J, Imtiaz S, Zarkin GA. Modeling the impact of COVID‐19 pandemic‐driven increases in alcohol consumption on health outcomes and hospitalization costs in the United States. Addiction. 2022. 10.1111/add.16018
REFERENCES
- 1. Esterwood E, Saeed SA. Past epidemics, natural disasters, COVID19, and mental health: learning from history as we deal with the present and prepare for the future. Psychiatry Q. 2020;91:1121–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu P, Liu X, Fang Y, Fan B, Fuller CJ, Guan Z, et al. Alcohol abuse/dependence symptoms among hospital employees exposed to a SARS outbreak. Alcohol Alcohol. 2008;43:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Moise IK, Ruiz MO. Hospitalizations for substance abuse disorders before and after hurricane Katrina: spatial clustering and area‐level predictors, New Orleans, 2004 and 2008. Prev Chronic Dis. 2016;13:E145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. North CS, Ringwalt CL, Downs D, Derzon J, Galvin D. Postdisaster course of alcohol use disorders in systematically studied survivors of 10 disasters. Arch Gen Psychiatry. 2011;68:173–80. [DOI] [PubMed] [Google Scholar]
- 5. Welch AE, Caramanica K, Maslow CB, Cone JE, Farfel MR, Keyes KM, et al. Frequent binge drinking five to six years after exposure to 9/11: findings from the world trade center health registry. Drug Alcohol Depend. 2014;140:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boscarino JA, Adams RE, Galea S. Alcohol use in New York after the terrorist attacks: a study of the effects of psychological trauma on drinking behavior. Addict Behav. 2006;31:606–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shield K, Manthey J, Rylett M, Probst C, Wettlaufer A, Parry CD, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. 2020;5:E51–61. [DOI] [PubMed] [Google Scholar]
- 8. Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet. 2019;394:781–92. [DOI] [PubMed] [Google Scholar]
- 9. Roberts A, Rogers J, Mason R, Siriwardena AN, Hogue T, Whitley GA, et al. Alcohol and other substance use during the COVID‐19 pandemic: a systematic review. Drug Alcohol Depend. 2021;229:109150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schmidt RA, Genois R, Jin J, Vigo D, Rehm J, Rush B. The early impact of COVID‐19 on the incidence, prevalence, and severity of alcohol use and other drugs: a systematic review. Drug Alcohol Depend. 2021;228:109065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McKetta S, Morrison CN, Keyes K. Trends in US alcohol consumption frequency during the first wave of the SARS‐CoV‐2 pandemic. Alcohol Clin Exp Res. 2021;45:773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nordeck CD, Riehm KE, Smail EJ, Holingue C, Kane JC, Johnson RM, et al. Changes in drinking days among United States adults during the COVID‐19 pandemic. Addiction. 2022;117:331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Barbosa C, Cowell AJ, Dowd WN. Alcohol consumption in response to the COVID‐19 pandemic in the United States. J Addict Med. 2021;15:341–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barbosa C, Dowd W, Barnosky A, Karriker‐Jaffe K. Alcohol consumption during the first year of the COVID‐19 pandemic in the United States: results from a nationally representative longitudinal survey. J Addict Med. 2022. 10.1097/ADM.0000000000001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Barbosa C, Dowd W, Karriker‐Jaffe K. How Has Drinking Behavior Changed During the COVID‐19 Pandemic? RTI International. 2021. Available from: https://www.rti.org/sites/default/files/fy21_covid_drinking_webinar_slides_final.pdf. Accessed 7 October 2021.
- 16. Barbosa C, Dowd WN, Aldridge AP, Timko C, Zarkin GA. Estimating long‐term drinking patterns for people with life‐time alcohol use disorder. Med Decis Making. 2019;39:765–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brennan A, Chick SE, Davies R. A taxonomy of model structures for economic evaluation of health technologies. J Health Econ. 2006;15:1295–310. [DOI] [PubMed] [Google Scholar]
- 18. Neumann PJ, Sanders GD, Russels LB, Siegel JE, Ganiats TG. Cost‐effectiveness in Health and Medicine 2nd ed. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 19. World Health Organization . International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 20. Neumann PJ, Cohen JT. Qalys in 2018—advantages and concerns. JAMA. 2018;319:2473–4. [DOI] [PubMed] [Google Scholar]
- 21. Substance Abuse and Mental Health Services Administration (SAMHSA) . Key substance use and mental health indicators in the United States: results from the 2019 National Survey on Drug Use and Health. Report no.: HHS Publication no. PEP20–07–01‐001, NSDUH Series H‐55. Rockville, MD: SAMHSA; 2020.
- 22. National Survey on Drug Use and Health (NSDUH) . 2019 NSDUH Detailed Tables Section 5 PE: Substance Use Disorder and Treatment Tables. 2019. [cited 2021 June 30]; Available from: https://www.samhsa.gov/data/sites/default/files/reports/rpt29394/NSDUHDetailedTabs2019/NSDUHDetTabsSect5pe2019.htm. Accessed 15 October 2020.
- 23. Grant B, Chu A, Sigman R, Amsbary M, Kali J, Sugawara Y, et al. National Epidemiologic Survey on Alcohol and Related Conditions‐III (NESARC‐III): Source and Accuracy Statement Rockville, MD: National Institute on Alcohol Abuse and Alcoholism; 2014. [Google Scholar]
- 24. Dawson DA, Stinson FS, Chou SP, Grant BF. Three‐year changes in adult risk drinking behavior in relation to the course of alcohol‐use disorders. J Stud Alcohol Drugs. 2008;69:866–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Grant BF, Stinson FS, Dawson DA, Chou SP, Ruan WJ, Pickering RP. Co‐occurrence of 12‐month alcohol and drug use disorders and personality disorders in the United States: results from the National Epidemiologic Survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:361–8. [DOI] [PubMed] [Google Scholar]
- 26. Humphreys K, Moos RH, Cohen C. Social and community resources and long‐term recovery from treated and untreated alcoholism. J Stud Alcohol. 1997;58:231–8. [DOI] [PubMed] [Google Scholar]
- 27. Moos R, Cronkite R, Finney J. Health and Daily Living Form Manual. Redwood City, CA: Mind Garden; 1992. [Google Scholar]
- 28. Kirkpatrick S, Gelatt CD Jr, Vecchi MP. Optimization by simulated annealing. Science. 1983;220:671–80. [DOI] [PubMed] [Google Scholar]
- 29. Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, et al. Epidemiology of DSM‐5 alcohol use disorder: results from the national epidemiologic survey on alcohol and related conditions III. JAMA Psychiatry. 2015;72:757–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. World Health Organization . Global Status Report on Alcohol and Health. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 31. Global Burden of Disease 2016 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease study 2016. Lancet. 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwarzinger M, Thiébaut SP, Baillot S, Mallet V, Rehm J. Alcohol use disorders and associated chronic disease—a national retrospective cohort study from France. BMC Public Health. 2017;18:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rehm J, Probst C, Shield KD, Shuper PA. Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modelling strategy for quantifying the effect. Popul Health Metr. 2017;15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rehm J, Sherk A, Shield KD, Gmel G. Risk Relations Between Alcohol Use and Non‐Injury Causes of Death. Toronto, ON: Centre for Addiction and Mental Health; 2017. [Google Scholar]
- 35. Centers for Disease Control and Prevention (CDC) . About Underlying Cause of Death, 1999–2015 Atlanta, GA, CDC; 2017. [Google Scholar]
- 36. Centers for Disease Control and Prevention (CDC) . WONDER Online Databases Utilize a Rich Ad‐Hoc Query System for the Analysis of Public Health Data Atlanta, GA: CDC; 2017. [Google Scholar]
- 37. Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J. Contribution of alcohol use disorders to the burden of dementia in France 2008‐2013: a nationwide retrospective cohort study. Lancet Public Health. 2018;3:e124–32. [DOI] [PubMed] [Google Scholar]
- 38. Roerecke M, Rehm J. Alcohol use disorders and mortality—a systematic review and meta‐analysis. Addiction. 2013;108:1562–78. [DOI] [PubMed] [Google Scholar]
- 39. Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project National Inpatient Sample. Rockville, MD. 2014. Available from: www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 29 August 2018.
- 40. Agency for Healthcare Research and Quality . Healthcare Cost and Utilization Project National Readmissions Database. Rockville, MD. 2014. Available from: www.hcup-us.ahrq.gov/nrdoverview.jsp. Accessed 29 August 2018.
- 41. Agency for Healthcare Research and Quality . Medical Expenditure Panel Survey, Household Component Rockville, MD: Agency for Healthcare Research and Quality; 2014. [Google Scholar]
- 42. Bureau of Labor Statistics . Medical care commodities in U.S. city average, all urban consumers, not seasonally adjusted. 2019. Available from: https://beta.bls.gov/dataViewer/view/timeseries/CUUR0000SAM1. Accessed 16 June 2021.
- 43. Grant BF, Chu A, Sigman R, Amsbary M, Kali J, Sugawara Y, et al. National Epidemiologic Survey on Alcohol and Related Conditions‐III (NESARC‐III). Source and Accuracy Statement. Rockville, MD: National Institute on Alcohol Abuse and Alcoholism. 2014. [Google Scholar]
- 44. Barbosa C, Bray JW, Dowd WN, Barnosky A, Wittenberg E. SF‐6D utility scores for alcohol use disorder status and alcohol consumption risk levels in the US population. Addiction. 2021;116:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wittenberg E, Bray JW, Gebremariam A, Aden B, Nosyk B, Schackman BR. Joint utility estimators in substance use disorders. Value Health. 2017;20:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16:231–50. [DOI] [PubMed] [Google Scholar]
- 47. Bennett C, Manuel DG. Reporting guidelines for modelling studies. BMC Med Res Methodol. 2012;12:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes 3rd ed. Oxford, UK: Oxford University Press; 2005. [Google Scholar]
- 49. Nuijten MJ, Pronk MH, Brorens MJ, Hekster YA, Lockefeer JH, de Smet PA, et al. Reporting format for economic evaluation. PharmacoEcon. 1998;14:259–68. [DOI] [PubMed] [Google Scholar]
- 50. Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM, et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. 2017;390:1345–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. World Health Organization . Global Status Report on Alcohol and Health Geneva, Switzerland: World Health Organization; 2014. p. 52. [Google Scholar]
- 52. Rehm J, Dawson D, Frick U, Gmel G, Roerecke M, Shield KD, et al. Burden of disease associated with alcohol use disorders in the United States. Alcohol Clin Exp Res. 2014;38:1068–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sacks JJ, Gonzales KR, Bouchery EE, Tomedi LE, Brewer RD. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49:e73–9. [DOI] [PubMed] [Google Scholar]
- 54. Cahan E Alcohol‐related liver disease surging. 2021. Available from: https://www.columbian.com/news/2021/feb/23/alcohol-related-liver-disease-surging/. Accessed 20 November 2021.
- 55. Noguchi Y. Sharp, ‘off the charts’ rise in alcoholic liver disease among young women. 2021. Available from: https://www.npr.org/sections/health‐shots/2021/03/16/973684753/sharp‐off‐the‐charts‐rise‐in‐alcoholic‐liver‐disease‐among‐young‐women?utm_source=facebook.com&utm_campaign=npr&utm_term=nprnews&utm_medium=social&fbclid=IwAR2z7pNV9sOHoyoA_m6FmPPV3MibEgq_qCmq_N0Ed_PKAXw82Sc0kE9QrVs. Accessed 20 November 2021.
- 56. Bittermann T, Mahmud N, Abt P. Trends in liver transplantation for acute alcohol‐associated hepatitis during the COVID‐19 pandemic in the US. JAMA Netw Open. 2021;4:e2118713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Anderson MS, Valbuena VS, Brown CS, Waits SA, Sonnenday CJ, Englesbe M, et al. Association of COVID‐19 with new waiting list registrations and liver transplantation for alcoholic hepatitis in the United States. JAMA Netw Open. 2021;4:e2131132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barbosa C, Dowd W, Karriker‐Jaffe K. How Has Drinking Behavior Changed During the COVID‐19 Pandemic? RTI Int. 2021. Available from: https://www.rti.org/sites/default/files/fy21_covid_drinking_webinar_slides_final.pdf. Accessed 7 October 2021.
- 59. Acuff SF, Strickland JC, Tucker JA, Murphy JG. Changes in alcohol use during COVID‐19 and associations with contextual and individual difference variables: a systematic review and meta‐analysis. Psychol Addict Behav. 2022;36:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. White AM. Gender differences in the epidemiology of alcohol use and related harms in the United States. Alcohol Res. 2020;40(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. White AM, Castle IP, Hingson RW. Using death certificates to explore changes in alcohol‐related mortality in the United States, 1999 to 2017. Alcohol Clin Exp Res. 2020;44:178–87. [DOI] [PubMed] [Google Scholar]
- 62. Kim EJ, Marrast L, Conigliaro J. COVID‐19: magnifying the effect of health disparities. J Gen Intern Med. 2020;2:2441–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zemore SE, Karriker‐Jaffe KJ, Mulia N, Kerr WC, Ehlers CL, Cook WK, et al. The future of research on alcohol‐related disparities across US racial/ethnic groups: a plan of attack. J Stud Alcohol Drugs. 2018;79:7–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huhn AS, Strain EC, Jardot J, Turner G, Bergeria CL, Nayak S, et al. Treatment disruption and childcare responsibility as risk factors for drug and alcohol use in persons in treatment for substance use disorders during the COVID‐19 crisis. J Addict Med. 2022;16:e8–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Whaley CM, Pera MF, Cantor J, Changr J, Velascor J, Haggr HK, et al. Changes in health services use among commercially insured US populations during the COVID‐19 pandemic. JAMA Netw Open. 2020;3:e2024984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Substance Abuse and Mental Health Services Administration . Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication no. PEP21–07–01‐003, NSDUH Series H‐56). 2021. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration. Available from: https://www.samhsa.gov/data/. Accessed 15 November 2021.
- 67. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM‐IV) Falls Church, VA: American Psychiatric Association; 1994. [Google Scholar]
- 68. American Psychiatric Association . Diagnostic and statistical manual of mental disorders (DSM‐5®) Falls Church, VA: American Psychiatric Association; 2013. [Google Scholar]
- 69. Bartoli F, Carrà G, Crocamo C, Clerici M. From DSM‐IV to DSM‐5 alcohol use disorder: an overview of epidemiological data. Addict Behav. 2015;41:46–50. [DOI] [PubMed] [Google Scholar]
- 70. Pollard MS, Tucker JS, Green HD Jr. Changes in adult alcohol use and consequences during the COVID‐19 pandemic in the US. JAMA Netw Open. 2020;3:e2022942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Minhas M, Belisario K, Gonzalez‐Roz A, Halladay J, Morris V, Keough M, et al. Is talk cheap? Correspondence between self‐attributions about changes in drinking and longitudinal changes in drinking during the 2019 coronavirus pandemic. Alcohol Clin Exp Res. 2021;45:2560–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Stockwell T, Donath S, Cooper‐Stanbury M, Chikritzhs T, Catalano P, Mateo C. Under‐reporting of alcohol consumption in household surveys: a comparison of quantity‐frequency, graduated‐frequency and recent recall. Addiction. 2004;99:1024–33. [DOI] [PubMed] [Google Scholar]
- 73. Livingston M, Callinan S. Underreporting in alcohol surveys: whose drinking is underestimated? J Stud Alcohol Drugs. 2015;76:158–64. [PubMed] [Google Scholar]
- 74. Del Boca FK, Darkes J. The validity of self‐reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98:1–12. [DOI] [PubMed] [Google Scholar]
- 75. Slater M, Alpert H. Apparent Per Capita Alcohol Consumption: National, State, and Regional Trends, 1977–2020. Surveillance Report #119. National Institutes of Health. 2022. Available from: https://pubsniaaanihgov/Publications/surveillance119/CONS20htm. Accessed 29 June 2022.
- 76. Jackson KM, Merrill JE, Stevens AK, Hayes KL, White HR. Changes in alcohol use and drinking context due to the COVID‐19 pandemic: a multimethod study of college student drinkers. Alcohol Clin Exp Res. 2021;45:752–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jaffe AE, Kumar SA, Ramirez JJ, DiLillo D. Is the COVID‐19 pandemic a high‐risk period for college student alcohol use? A comparison of three spring semesters. Alcohol Clin Exp Res. 2021;45:854–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. National Institute on Alcohol Abuse and Alcoholism. Alcohol Sales During the COVID‐19 Pandemic. 2022. Available from: https://pubs.niaaa.nih.gov/publications/surveillance-covid-19/COVSALES.htm. Accessed 29 June 2022.
- 79. Gilhooly MLM. Reduced drinking with age: is it normal? Addict Res Theor. 2005;13:267–80. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Model Parameter Details and Methods
Appendix B. Additional Model Results, Life‐time Follow‐Up
Appendix C. Additional Model Results, 5‐year Follow‐Up
Appendix D. Assessing the Impact of Increased Mortality for all Non‐Alcohol‐Related Causes during the COVID‐19 Pandemic


