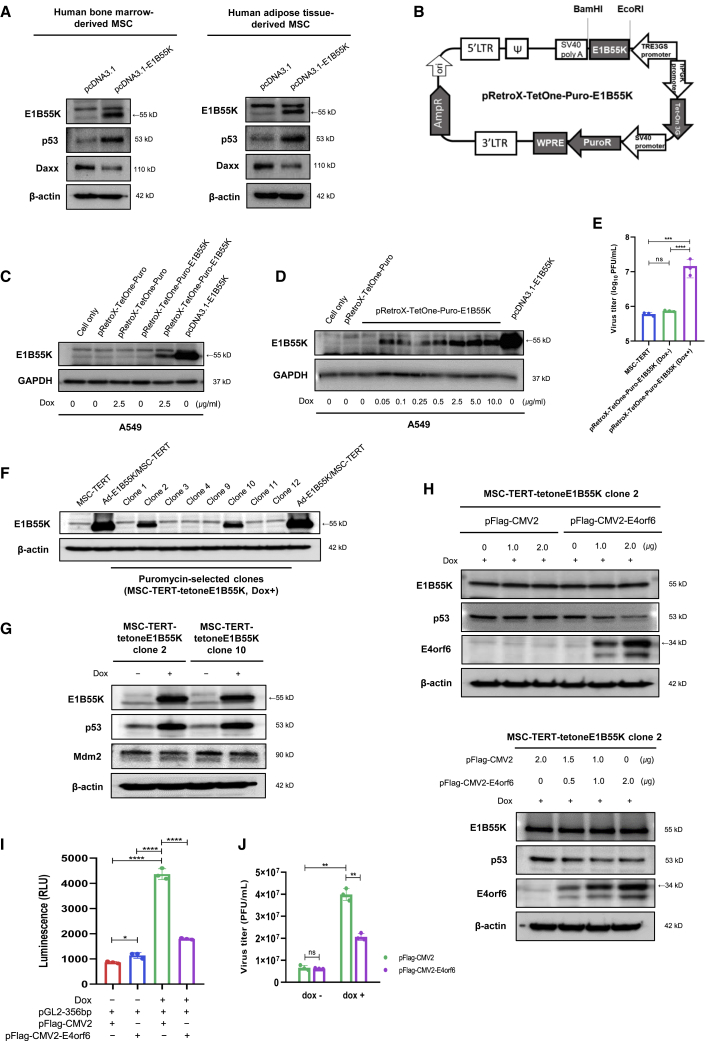
Figure 4.
E1B55K alone increases p53 accumulation rather than p53 degradation
(A) Human bone marrow-derived MSCs (left) and human adipose tissue-derived MSCs (right) were transfected with 2 μg of pcDNA3.1-Hygro or pcDNA3.1-Hygro-E1B55K plasmid. After 48 h, protein expression was analyzed by western blot. (B) Schematic structure of pRetroX-TetOne-Puro-E1B55K. (C) A549 was transfected with 2 μg of pRetroX-TetOne-Puro, pRetroX-TetOne-Puro-E1B55K, or pcDNA3.1-E1B55K, and then cells were treated with or without 2.5 μg/mL doxycycline. After 48 h, cells were lysed for detection of E1B55K expression levels by western blot. (D) Induction of E1B55K expression in A549 in the presence of various concentrations of doxycycline. A549 was transfected with 2 μg of pRetroX-TetOne-Puro, pRetroX-TetOne-Puro-E1B55K, or pcDNA3.1-E1B55K, and then, only cells transfected with pRetroX-TetOne-Puro-E1B55K were treated with doxycycline at indicated concentrations. After 48 h, cells were lysed for detection of E1B55K expression levels by western blot. (E) Measuring virus production levels in MSC-TERT transfected with pRetro-X-TetOne-Puro-E1B55K. Cells were transfected with 1 μg of pRetroX-TetOne-Puro-E1B55K, and then infected with 100 MOIs of Ad-3484-RFP. After 4 h of infection, cells were treated with or without 0.5 μg/mL doxycycline. After 48 h, all virus particles released from the cells were collected and the amount of infectious virus particles was calculated. Error bars are mean ± SD from three independent experiments: ∗∗∗p < 0.001; ∗∗∗∗p < 0.0001; ns, not significant. (F) Screening for inducible expression of E1B55K in puromycin-resistant clones in the presence of 0.5 μg/mL doxycycline. Positive clones for inducible expression of E1B55K were identified using MSC-TERT as a negative control and MSC-TERT infected with Ad-E1B55K (Ad-E1B55K/MSC-TERT) as a positive control. Ad-E1B55K/MSC-TERT was loaded on both ends to more clearly confirm the expression of E1B55K. (G) Protein expression in MSC-TERT-tetoneE1B55K clones treated with or without doxycycline for 36–48 h. (H) MSC-TERT-tetoneE1B55K clone 2 was transfected with pFLAG-CMV2 or pFLAG-CMV2-E4orf6 plasmid at indicated concentrations. Then, doxycycline was added to induce E1B55K expression. After 48 h, cells were lysed and protein expression levels were detected by western blot. (I) Measuring p53 promoter activity in MSC-TERT-tetoneE1B55K clone 2 by luciferase reporter assay. Cells were cotransfected with pGL2-356bp and pFLAG-CMV2 or pGL2-356bp and pFLAG-CMV2-E4orf6 (1 μg of each plasmid). Then, cells were treated with or without doxycycline for 48 h to induce E1B55K expression. The transcriptional activity of p53 was shown based on the measured firefly luciferase activities. Error bars are mean ± SD from three independent experiments: ∗p < 0.05, ∗∗∗∗p < 0.0001. (J) Measurement of virus production in MSC-TERT-tetoneE1B55K clone 2. Cells were transfected with 1 μg of pFLAG-CMV2 or pFLAG-CMV2-E4orf6 and then infected with 100 MOIs of Ad-3484-shHSP27-shTGF-β1. After 4 h of infection, cells were treated with or without doxycycline. After 48 h, all virus particles released from the cells were collected and the amount of infectious virus particles was calculated. Error bars are mean ± SD from three independent experiments: ∗∗p < 0.01; ns, not significant.