Abstract
This study aimed to evaluate the inhibitory effects of phenolic‐rich extracts from acerola (Malpighia emarginata D.C., PEA), cashew apple (Anacardium occidentale L., PEC) and mango (Mangifera indica L., PEM) by‐products on distinct enterotoxigenic Escherichia coli (ETEC) strains. The capability of PEA and PEC of impairing various physiological functions of ETEC strains was investigated with multiparametric flow cytometry. Procyanidin B2, myricetin and p‐coumaric acid were the major phenolic compounds in PEA, PEC and PEM, respectively. PEA and PEC had lower minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) (MIC: 31·25 mg ml−1; MBC: 62·5 mg ml−1) on ETEC strains than PEM (MIC and MIC: >1000 mg ml−1). PEA and PEC (15·6, 31·2, 62·5 mg ml−1) caused viable count reductions (P < 0·05) on ETEC strains after 24 h of exposure, notably the ≥3 log reductions caused by 62·5 mg ml−1. The 24 h exposure of ETEC strains to PEA and PEC (31·2, 62·5 mg ml−1) led to high sizes of cell subpopulations with concomitant impairments in cell membrane polarization and permeability, as well as in enzymatic, respiratory and efflux activities. PEA and PEC are effective in inhibiting ETEC through a multi‐target action mode with disturbance in different physiological functions.
Keywords: antibacterial properties, cell damage, enterotoxigenic Escherichia coli , extracts, fruit
Significance and Impact of the Study: Enterotoxigenic Escherichia coli (ETEC) is one of the main causes of diarrhoea in humans and animals. The increasing resistance of ETEC strains to clinically relevant antibiotics has prompt the necessity to search for novel substances with inhibitory efficacy. This is the first study showing the capability of phenolic‐rich extracts of acerola and cashew apple by‐products causing the inhibition of distinct ETEC strains, with disturbance of various physiological functions, indicating these substances as potential antimicrobials to be exploited in solutions to control ETEC in humans and animals.
Introduction
Escherichia coli is a well‐known Gram‐negative bacterium belonging to Enterobacteriaceae family. The primary E. coli habitat is the intestinal tract of human and warm‐blooded animal, where it is usually part of the normal microbiota as a commensal bacterium. However, several E. coli pathotypes can cause intestinal diseases in both healthy and immunocompromised individuals (Gomes et al. 2016; Jang et al. 2017). Enterotoxigenic E. coli (ETEC) pathotype is one of the most widespread causes of diarrhoea in humans and animals worldwide. The presence of ETEC in the environment, food and water allows this bacterium to achieve the intestine via the oral route. Fimbrial or non‐fimbrial adhesins are involved in ETEC adherence to the intestinal mucosa epithelium where it can secrete enterotoxins, that is, heat‐labile toxin and heat‐stable toxins (STs), leading to watery diarrhoea, dehydration and even death (Dubreuil et al. 2016; Gomes et al. 2016).
The incidence of human diarrhoea caused by ETEC in developing countries occurs typically in the earlier 5 years of life, as well as in immunocompromised individuals and travellers (Gomes et al. 2016; Khalil et al. 2018). Moreover, post‐weaning diarrhoea caused by ETEC is a common disease in farm animals, especially in piglets, resulting in decreased weight gain and death. Diarrheal illnesses in animals have important negative impacts on the agro‐food industry causing great economic losses (Dubreuil et al. 2016; Luppi 2017). The resistance to clinically relevant antibiotics has been progressively reported in ETEC strains, being an important concern to control ETEC infections in humans and animals (Luppi 2017; Davin‐Regli et al. 2019). The search for substances or compounds with strong antibacterial effects and capability of acting on distinct sites of ETEC cells should be a research focus (Khalil et al. 2018; Dubreuil 2020).
Phenolic compounds are chemically characterized by the presence of an aromatic structure and multiple hydroxyl groups in their molecules, which have been putatively associated with their potential antimicrobial properties (Khalifa et al. 2018; Lima et al. 2019). Acerola (Malpighia emarginata D.C.), cashew apple (Anacardium occidentale L.) and mango (Mangifera indica L.) are tropical fruit abundantly found in Brazil and rich sources of phenolic compounds (Asif et al. 2016; Duarte et al. 2017; Belwal et al. 2018). The agro‐industrial processing of these fruit generates high amounts of by‐products mostly composed of peel and/or seed remnants with higher contents of phenolic compounds than fruit pulps (Silva et al. 2014; Asif et al. 2016; Duarte et al. 2017; Batista et al. 2018; Araújo et al. 2020). This fact illustrates the necessity to develop strategies to recover the phenolic compounds from these fruit by‐products and transform them into products with value‐added applications to other industries, which could also reduce the environmental concerns regarding the agro‐industrial waste disposal (Fierascu et al. 2020). Some previous studies have shown the efficacy of phenolic compounds and phenolic‐rich extracts from fruit to inhibit different pathogenic bacteria and modulate the antibiotic bacterial resistance (Diniz‐Silva et al. 2017; Sanhueza et al. 2017; Lima et al. 2019). However, investigations on the inhibitory effects of phenolic‐rich extracts from fruit or their by‐products on ETEC are still deficient.
This study hypothesized that phenolic‐rich extracts from acerola, cashew apple and mango by‐products could exert antibacterial properties against ETEC. To test this hypothesis, the effects of these extracts on the growth/survival of distinct ETEC strains, as well as the capability of the more active extracts of impairing various bacterial physiological functions as events underlying their inhibitory effects on target ETEC strains were evaluated.
Results and discussion
The phenolic compounds determined in PEA, PEC and PEM are shown in Table 1. Procyanidin B2, catechin and hesperetin were the phenolic compounds found in the highest contents in PEA. Myricetin was the major phenolic compound in PEC, followed by procyanidin B2 and catechin. p‐coumaric acid and hesperetin were the major phenolic compounds in PEM, followed by quercetin 3‐glucoside. PEM had the highest diversity of the determined phenolic compounds (23), followed by PEA (19) and PEC (10). The decreasing rank based on the summed total content of the determined phenolic compounds was PEA (244·0 ± 11·6 mg 100 g−1)>PEM (157·3 ± 18·8 mg g−1)>PEC (93·0 ± 0·7 mg 100 g−1). Previous studies have reported antibacterial effects of catechin (Sinsinwar and Vadivel 2020), p‐coumaric acid (Bag and Chattopadhyay 2017; Ojha and Patil 2019) and procyanidin B2 (Alejo‐Armijo et al. 2017) on some pathogenic bacteria.
Table 1.
Phenolic compounds identified (average ± standard deviation) in phenolic‐rich extracts from acerola (PEA), cashew apple (PEC) and mango (PEM) by‐products
| Phenolic compounds | PEA (mg 100 g−1) | PEC (mg 100 g−1) | PEM (mg 100 g−1) |
|---|---|---|---|
| Anthocyanidins | |||
| Cyanidin 3,5‐diglucoside | 0·7 (±0·2) | <LOD | <LOD |
| Malvidin 3,5‐diglucoside | <LOD | <LOD | 0·9 (±0·2) |
| Malvidin 3‐glucoside | 8·9 (±0·9) | <LOD | <LOD |
| Pelargonidin 3‐glucoside | 1·3 (±0·3)a | 2·4 (±0·1)b | <LOD |
| Pelargonidin 3,5‐diglucoside | <LOD | <LOD | 1·3 (±0·0) |
| Petunidin 3‐glucoside | 14·7 (±1·7) | <LOD | <LOD |
| Flavanols | |||
| Catechin | 35·1 (±0·4)a | 10·5 (±0·2)b | 2·7 (±0·2)c |
| Epicatechin | <LOD | <LOD | 1·3 (±0·1) |
| Epicatechin gallate | <LOD | <LOD | 0·7 (±0·3) |
| Epigallocatechin gallate | 3·8 (±1·0)a | <LOD | 2·4 (±0·1)b |
| Procyanidin A2 | 3·8 (±0·0)a | <LOD | 0·9 (±0·2)b |
| Procyanidin B1 | 20·9 (±0·3)a | 0·3 (±0·1)c | 15·9 (±2·3)b |
| Procyanidin B2 | 39·1 (±0·7)a | 17·5 (±0·5)b | 9·0 (±0·3)c |
| Flavonol | |||
| Kampferol 3‐glucoside | 12·0 (±0·2)a | 0·4 (±0·0)b | 0·7 (±0·1)b |
| Myricetin | <LOD | 52·0 (±0·4)a | 0·6 (±0·2)b |
| Quercetin 3‐glucoside | 15·8 (±0·1)b | 3·2 (±0·0)c | 18·1 (±0·9)a |
| Rutin | 1·2 (±0·0)b | 2·8 (±0·0)b | 6·9 (±0·8)a |
| Flavanones | |||
| Hesperetin | 32·6 (±0·6)a | 3·5 (±0·0)b | 34·8 (±1·7)a |
| Naringenin | 16·7 (±0·3)a | <LOD | 1·1 (±0·2)b |
| Hydroxybenzoic acids | |||
| Gallic acid | <LOD | <LOD | 0·2 (±0·0) |
| Syringic acid | <LOD | <LOD | 1·6 (±0·2) |
| Hydroxycinnamic acids | |||
| Caffeic acid | 0·9 (±0·1)a | < LOD | 1·3 (±0·3)a |
| Caftaric acid | 19·4 (±3·6)a | 0·3 (±0·1)b | 3·6 (±0·4)b |
| Chlorogenic acid | 6·5 (±1·1)a | <LOD | 1·4 (±0·2)b |
| p‐Coumaric acid | <LOD | <LOD | 34·9 (±5·6) |
| Stilbenes | |||
| cis‐Resveratrol | 2·5 (±0·2)b | <LOD | 14·3 (±2·7)a |
| trans‐Resveratrol | 8·1 (±0·2)a | <LOD | 3·0 (±0·2)b |
| Sum of contents of determined phenolic compounds | 244·0 | 93·0 | 157·3 |
<LOD: Below the limit of detection.
Different letters in the same row denote significant differences (P < 0·05), based on Kruskal–Wallis or Mann–Whitney U test.
Gallic acid (limit of detection (LOD) 0·001 mg 100 g−1 and limit of quantification (LOQ) 0·001 mg 100 g−1), syringic acid (LOD 0·008 mg 100 g−1 and LOQ 0·012 mg 100 g−1), cis‐resveratrol (LOD 0·008 mg 100 g−1 and LOQ 0·010 mg 100 g−1), hesperidin (LOD 0·012 mg 100 g−1 and LOQ 0·017 mg 100 g−1), naringenin (LOD 0·016 mg 100 g−1 and LOQ 0·022 mg 100 g−1), epicatechin (LOD 0·006 mg 100 g−1 and LOQ 0·007 mg 100 g−1), catechin (LOD 0·006 mg 100 g−1 and LOQ 0·008 mg 100 g−1), epicatechin‐gallate (LOD 0·006 mg 100 g−1 and LOQ 0·007 mg 100 g−1), epigallocatechin‐gallate (LOD 0·004 mg 100 g−1 and LOQ 0·005 mg 100 g−1), procyanidin A2 (LOD 0·006 mg 100 g−1 and LOQ 0·007 100 g−1), procyanidin B1 (LOD 0·001 mg 100 g−1 and LOQ 0·001 mg 100 g−1) and procyanidin B2 (LOD 0·004 mg 100 g−1 and LOQ 0·006 mg 100 g−1), trans‐caftaric acid (LOD 0·009 mg 100 g−1 and LOQ 0·001 100 g−1), ρ‐coumaric acid (LOD 0·010 mg 100 g−1 and LOQ 0·012 mg 100 g−1), chlorogenic acid (LOD 0·011 mg 100 g−1 and LOQ 0·015 mg 100 g−1), caffeic acid (LOD 0·001 mg 100 g−1 and LOQ 0·001 mg 100 g−1), trans‐resveratrol (LOD 0·004 mg 100 g−1 and LOQ 0·006 mg 100 g−1), kaempferol (LOD 0·010 mg 100 g−1 and LOQ 0·012 mg 100 g−1), quercetin 3‐glucoside (LOD 0·011 mg 100 g−1 and LOQ 0·014 mg 100 g−1), rutin (LOD 0·008 mg 100 g−1 and LOQ 0·010 mg 100 g−1), myricetin (LOD 0·005 mg 100 g−1 and LOQ 0·007 mg g−1), malvidin 3‐glucoside (LOD 0·085 mg 100 g−1 and LOQ 0·141 mg 100 g−1), cyanidin 3‐glucoside (LOD 0·011 mg 100 g−1 and LOQ 0·015 mg 100 g−1), malvidin 3,5‐diglucoside (LOD 0·024 mg 100 g−1 and LOQ 0·028 mg 100 g−1), cyanidin 3,5‐diglucoside (LOD 0·007 mg 100 g−1 and LOQ 0·008 mg 100 g−1), pelargonidin 3‐glucoside (LOD 0·006 mg 100 g−1 and LOQ 0·008 mg 100 g−1), delphinidin 3‐glucoside (LOD 0·017 mg 100 g−1 and LOQ 0·022 mg 100 g−1), pelargonidin 3,5‐diglucoside (LOD 0·005 mg 100 g−1 and LOQ 0·005 mg 100 g−1), petunidin 3‐glucoside (LOD 0·010 mg 100 g−1 and LOQ 0·012 mg 100 g−1).
A variety of major phenolic compounds have been reported in acerola (e.g. catechin, myricetin, peonidin 3‐glucoside, hesperidin and procyanidin B2) (Batista et al. 2018; Belwal et al. 2018; Gomes et al. 2020) and cashew apple by‐product (e.g. salicylic acid, myricetin, epicatechin and rutin) (Gordon et al. 2012; Batista et al. 2018). Mangiferin, epicatechin‐gallate and epigallocatechin‐gallate were previously identified as the major phenolic compounds in mango peels (Asif et al. 2016; Coelho et al. 2019). The extraction procedure, season, ripening stage and plant cultivar could be factors affecting the types and contents of phenolic compounds in different fruit samples belonging to a same plant species (Gordon et al. 2012; Haminiuk et al. 2012; de Albuquerque et al. 2019; Lima et al. 2019).
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of PEA, PEC and PEM on distinct target ETEC strains were determined as an initial screening step to evaluate their antibacterial properties. MIC and MBC of PEA, PEC and PEM were similar against the distinct target ETEC strains. Both PEA and PEC had MIC and MBC of 31·25 and 62·5 mg ml−1, respectively. PEM had MIC and MBC of >1000 mg ml−1, indicating weak inhibitory effects on target strains (Van Vuuren 2008; Diniz‐Silva et al. 2017). Considering the MIC and MBC found for the tested extracts, only PEA and PEC were selected for inclusion in further experiments.
An early study reported MIC of 50 mg ml−1 for a methanol extract from pomegranate (Punica granatum L.) by‐product on E. coli (Gullon et al. 2016). Available literature has shown superior inhibitory effects of phenolic compounds commonly found in fruit on Gram‐positive bacteria, although these compounds have also exerted inhibitory effects on distinct Gram‐negative bacteria, including E. coli (Díaz‐Gómez et al. 2014; Paz et al. 2015; Coman et al. 2018; Lima et al. 2019). MIC and MBC of PEA, PEC and PEM on target ETEC strains were not correlated (P ≥ 0·05) with total summed content of determined phenolic compounds in each extract. It indicates that the phenolic compound profile of PEA, PEC and PEM could affect the intensity of their inhibitory effects on target ETEC strains rather than the total summed content of determined phenolic compounds.
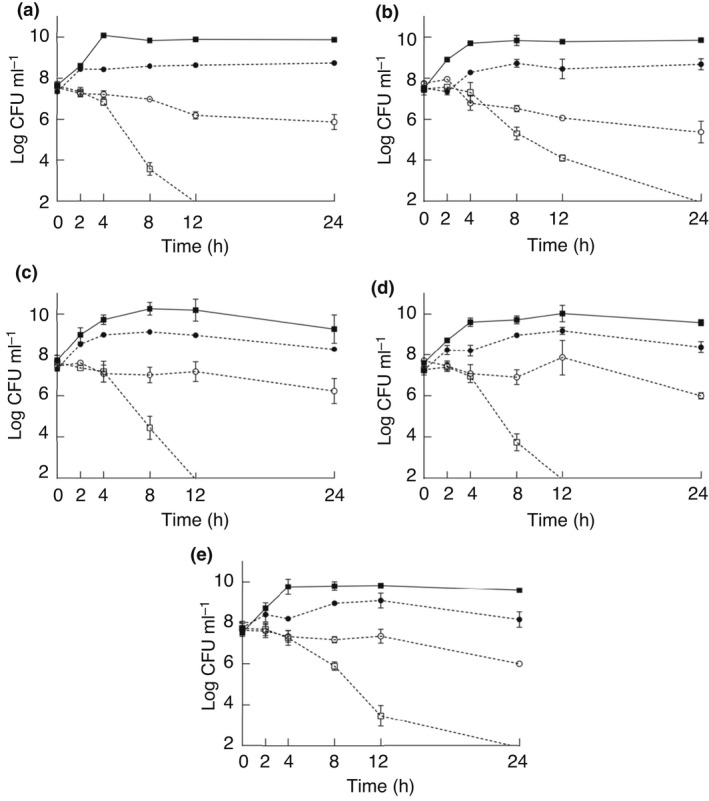
The effects of PEA and PEC (MIC/2: 15·6 mg ml−1, MIC: 31·2 mg ml−1 and MBC: 62·5 mg ml−1) on the viable counts of target ETEC strains are shown in Figs 1 and 2, respectively. PEA and PEC caused significant viable count reductions during the 24h of exposure (P < 0·001) regardless of the tested extract concentration and ETEC strain. Each PEA and PEC at a same concentration caused similar reductions (P ≥ 0·05) in viable counts of the distinct ETEC strains during the 24 h exposure. Viable count reductions of ≥5 log (i.e., ≥99·999% viable initial count reduction) were caused by 62·5 mg ml−1 of PEA on ETEC ECL20898 after 24 h (Fig. 1d). Viable count reductions of ≥5 log were caused by 62·5 mg ml−1 of PEC on ETEC ECL19790, ETEC ECL 23141 and ETEC ECL 20898 (Fig. 2a,c,d) after 12 h, as well as on ETEC ECL20440 and ETEC ECL21858 after 24 h (Fig. 2b,e). Viable count reductions of ≥3 log (i.e., ≥99·9% viable initial count reduction) were caused by 62·5 mg ml−1 of PEA on ETEC ECL19790, ETEC ECL20440, ETEC ECL231441 and ETEC ECL21858 after 24 h (Fig. 1a–c,e).
Figure 1.
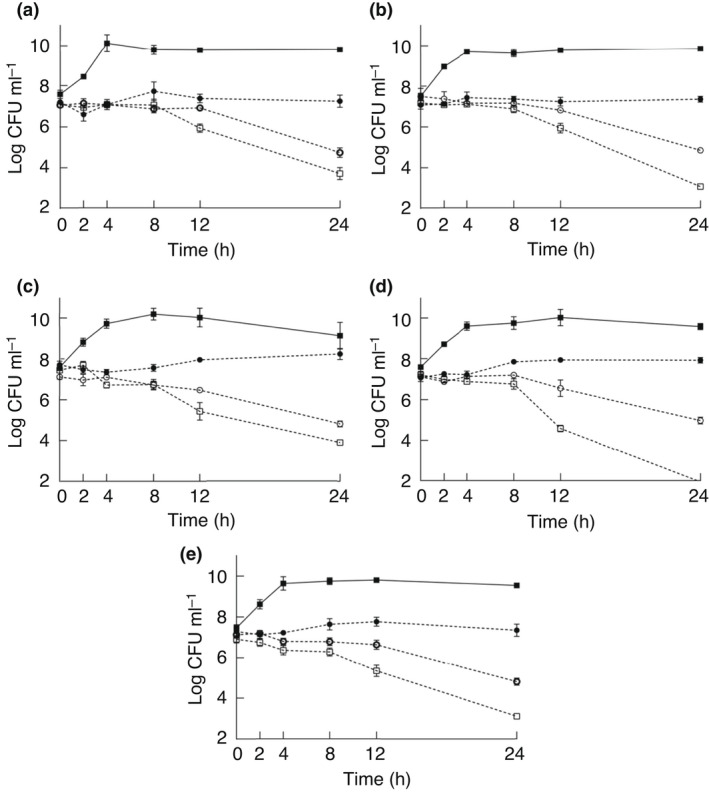
Viable counts of enterotoxigenic Escherichia coli ECL 19790 (a), ECL 20440 (b), ECL 23141 (c), ECL 20898 (d) and ECL 21858 (e) as a function of the exposure to different concentrations of phenolic‐rich extract from acerola by‐products (PEA). 62·5 mg ml−1 ( ), 31·2 mg ml−1 (
), 31·2 mg ml−1 ( ), 15·6 mg ml−1 (
), 15·6 mg ml−1 ( ), control (without exposure to extract) (
), control (without exposure to extract) ( ).
).
Figure 2.
Viable counts of enterotoxigenic Escherichia coli ECL 19790 (a), ECL 20440 (b), ECL 23141 (c), ECL 20898 (d) and ECL 21858 (e) as a function of the exposure to phenolic‐rich extract from cashew apple by‐products (PEC) in concentrations of 62·5 mg ml−1 ( ), 31·2 mg ml−1 (
), 31·2 mg ml−1 ( ), 15·6 mg ml−1 (
), 15·6 mg ml−1 ( ), control (without exposure to extract) (
), control (without exposure to extract) ( ).
).
The greatest viable count reductions caused by 31·2 mg ml−1 of PEA and PEC were observed on ETEC ECL20440 (2·98 and 2·75 log, respectively) after 24 h (Figs 1b and 2b), while the greatest viable count reductions caused by 15·6 mg ml−1 of PEA and PEC were on ETEC ECL19790 (1 log) and ETEC ECL20440 (0·43 log) after 2 h, respectively (Figs 1a and 2b).
The viable count reductions of tested ETEC strains at time zero (baseline) were compared with those after each pre‐established exposure time to PEA and PEC during the 24 h. The concentrations of 31·2 and 62·5 mg ml−1 of PEA caused significant viable count reductions after 12 h (P < 0·001) and 4 h (P = 0·010), respectively, while both 31·2 and 62·5 mg ml−1 of PEC caused significant viable count reductions (P < 0·001 and P = 0·010, respectively) after 4 h. The viable count reductions correlated (P < 0·001) with extract concentration (P = 0·785) and exposure time (P = 0·142).
The results showing the killing effects of PEA and PEC on distinct ETEC strains demonstrate their efficacy to inhibit this bacterium, notably the size of the viable count reductions caused by 62·5 mg ml−1 of PEA and PEC, which were always of ≥3 log after 24 h. Plant‐derived products capable of causing viable count reductions of ≥3 log have exerted strong bactericidal effects on target microorganism, while reductions of <3 log indicate bacteriostatic effects (LaPlante 2007; de Souza 2016; Lima and Souza 2020). The killing effects of phenolic‐rich extracts or phenolic compounds on E. coli have rarely been studied. An earlier study reported that gallic acid (4·5 mg ml−1) caused sharp viable count reductions in E. coli after 6 h of exposure, while catechin (5 mg ml−1) failed to cause significant reductions after 24 h of exposure (Díaz‐Gómez et al. 2014).
The effects of a 24 h exposure to 31·2 and 62·5 mg ml−1 of PEA and PEC on distinct physiological functions of three target ETEC strains, namely ETEC ECL20440, ETEC ECL23141 and ETEC ECL21858, were investigated with multiparametric flow cytometry (MFC) using five different fluorochromes (propidium iodide (PI), BOX, cFDA, CTC and EB) (Tables 2 and 3) to provide accurate information on cellular events underlying the reported viable count reductions. PEA and PEC caused disturbance (P < 0·001) of the various measured physiological functions regardless of the tested extract concentration and ETEC strain. However, the sizes of ETEC cell subpopulations with damage in measured physiological functions correlated (P < 0·001) with extract concentration (P = 0·748), which agree with the results of assays to measure viable count reductions over time.
Table 2.
Size of subpopulations (average ± standard deviation) of enterotoxigenic Escherichia coli strains (ECL 20440, ECL 23141 and ECL 21858) stained with PI, BOX, cFDA, CTC and EB after exposure to two different concentrations of phenolic‐rich extract from acerola by‐products (PEA, 31·25 and 62·50 mg ml−1)
| Cellular structure or function disruption | Enterotoxigenic E. coli strains | Treatments | ||
|---|---|---|---|---|
| Control (without exposure to extract) | PEA (31·25 mg ml−1) | PEA (62·50 mg ml−1) | ||
| Size of subpopulations (%) | ||||
| Permeabilized cells (PI+) | ECL 20440 | 5·6 ± 0.2Ac | 11·5 ± 2.7Bb | 28·7 ± 0.1Aa |
| ECL 23141 | 3·5 ± 0.5Bc | 17·5 ± 2.0Ab | 29·5 ± 1.8Aa | |
| ECL 21858 | 3·0 ± 1.8Bc | 10·0 ± 0.3Bb | 24·6 ± 1.9Ba | |
| Depolarized cells (BOX+) | ECL 20440 | 10·2 ± 0.4Ac | 24·5 ± 2.7Bb | 49·0 ± 3.5Ba |
| ECL 23141 | 8·1 ± 1.0Bc | 18·8 ± 1.5Cab | 25·2 ± 0.8Ca | |
| ECL 21858 | 7·6 ± 1.1Bc | 68·1 ± 2.3Ab | 86·9 ± 1.9Aa | |
| Cells without enzymatic activity (cF−) | ECL 20440 | 9·1 ± 0.6Ab | 90·5 ± 2.6Aa | 93·0 ± 1.4Aa |
| ECL 23141 | 5·0 ± 1.6Bb | 91·3 ± 4.7Aa | 96·4 ± 1.4Aa | |
| ECL 21858 | 8·9 ± 1.6Ac | 87·4 ± 1.7Ab | 95·1 ± 2.0Aa | |
| Cells without respiratory activity (CTC−) | ECL 20440 | 5·4 ± 1.1Ab | 58·0 ± 3.2Ca | 61·6 ± 2.3Ca |
| ECL 23141 | 6·0 ± 0.2Ac | 90·7 ± 1.5Ba | 73·8 ± 1.3Bb | |
| ECL 21858 | 7·7 ± 1.7Ab | 97·7 ± 3.7Aa | 99·3 ± 1.4Aa | |
| Cells with compromised efflux pump activity (EB+) | ECL 20440 | 10·5 ± 3.4Ac | 31·2 ± 4.3Bb | 45·6 ± 1.9Ba |
| ECL 23141 | 11·9 ± 0.8Ac | 27·5 ± 3.1Cb | 47·4 ± 1.0Ba | |
| ECL 21858 | 8·6 ± 0.7Bc | 47·6 ± 1.9Ab | 72·2 ± 2.5Aa | |
Different superscript lowercase letters (a–c) in the same row denote significant differences (P < 0·05), based on Kruskal–Wallis test. Different superscript capital letters (A–C) in the same column denote significant differences (P < 0·05) among different strains, for a same treatment and fluorescence stain, based on Kruskal–Wallis test.
Table 3.
Size of subpopulations (average ± standard deviation) of enterotoxigenic Escherichia coli strains (ECL 20440, ECL 23141 and ECL 21858) stained with PI, BOX, cFDA, CTC and EB after exposure to two different concentrations of phenolic‐rich extract from cashew apple by‐product (PEC, 31·25 and 62·50 mg ml−1)
| Cellular structure or function disruption | Enterotoxigenic E. coli strains | Treatments | ||
|---|---|---|---|---|
| Control (without exposure to extract) | PEC (31·25 mg ml−1) | PEC (62·50 mg ml−1) | ||
| Size of subpopulations (%) | ||||
| Permeabilized cells (PI +) | ECL 20440 | 3·8 ± 2.3Ac | 8·7 ± 0.3Ab | 14·92 ± 1.3Aa |
| ECL 23141 | 2·0 ± 0.9Ac | 3·4 ± 1.0Bb | 8·25 ± 1.0Ba | |
| ECL 21858 | 3·2 ± 1.5Ac | 3·9 ± 1.4Bb | 7·91 ± 1.4Ba | |
| Depolarized cells (BOX+) | ECL 20440 | 11·6 ± 0.4Ac | 53·7 ± 1.4Ab | 69·6 ± 3.4Aa |
| ECL 23141 | 6·1 ± 1.0Bb | 57·2 ± 3.4Aa | 59·7 ± 3.5Aa | |
| ECL 21858 | 5·5 ± 1.4Bc | 58·1 ± 4.7Aa | 47·0 ± 2.3Bb | |
| Cells without enzymatic activity (cF−) | ECL 20440 | 3·8 ± 0.2Cb | 19·4 ± 1.9Ba | 65·9 ± 5.1Ba |
| ECL 23141 | 7·4 ± 0.8Ac | 67·0 ± 2.2Ab | 84·4 ± 1.3Aa | |
| ECL 21858 | 4·9 ± 0.2Bc | 64·5 ± 1.8Ab | 80·3 ± 4.6Aa | |
| Cells without respiratory activity (CTC−) | ECL 20440 | 6·5 ± 0.4Ac | 32·3 ± 1.8Cb | 62·3 ± 2.4Ca |
| ECL 23141 | 5·5 ± 0.8Ab | 76·3 ± 4.7Ba | 74·6 ± 3.3Ba | |
| ECL 21858 | 6·9 ± 1.3Ab | 88·1 ± 3.1Aa | 83·1 ± 2.2Aa | |
| Cells with compromised efflux pump activity (EB+) | ECL 20440 | 8·5 ± 1.4Ac | 33·7 ± 2.6Cb | 56·8 ± 2.9Aa |
| ECL 23141 | 8·1 ± 0.2Ab | 51·9 ± 2.2Aa | 53·0 ± 3.5Aa | |
| ECL 21858 | 5·6 ± 0.1Bc | 42·8 ± 3.3Ba | 36·0 ± 5.0Bb | |
Different superscript lowercase letters (a–c) in the same row denote significant differences (P < 0·05), based on Kruskal–Wallis test. Different superscript capital letters (A–C) in the same column denote significant differences (P < 0·05) among different strains, for a same treatment and fluorescence stain, based on Kruskal–Wallis test.
The PI is an impermeant fluorochrome capable of interacting with only dead cells. Damaged cell membrane permits fluorescence after PI staining of nucleic acids (de Sousa Guedes and de Souza 2018; Wilkinson 2018; Barbosa et al. 2020). PEA caused higher sizes of cell subpopulations with damaged cell membranes (PI+) than PEC (P = 0·011). The exposure of ETEC strains to 62·5 and 31·2 mg ml−1 of PEA led to sizes of cell subpopulations with damaged membrane ranging from 24·6–29·5% and 10·0–17·5%, respectively (Table 2). The exposure to 62·5 and 31·2 mg ml−1 of PEC led to sizes of cell subpopulations with damaged membranes ranging from 7·9–14·9% and 3·4–8·7%, respectively (Table 3).
The BOX is a lipophilic molecule used to evaluate cell membrane potential. Functional bacterial cells with intact membranes are negatively charged and capable of eliminating anionic molecules (e.g., BOX) and only depolarized cells could allow BOX accumulation. Membrane potential of bacterial cells could indicate both membrane integrity and metabolic activity (Rezaeinejad and Ivanov 2011; de Sousa Guedes and de Souza 2018; Almeida et al. 2019). The exposure of ETEC strains to 62·5 and 31·25 mg ml−1 of PEA led to sizes of depolarized (BOX+) cell subpopulations ranging from 25·2 to 86·9% and 18·8 to 68·1%, respectively (Table 2). Exposure to 62·5 and 31·25 mg ml−1 of PEC led to sizes of depolarized cell subpopulations ranging from 47·0 to 69·6% and 53·7 to 58·1%, respectively (Table 3). The highest sizes (P = 0·011) of depolarized cell subpopulations were found for ETEC ECL 21858 following the exposure to PEA.
The cFDA is a non‐fluorescent substrate, although enzymatically active bacterial cells can hydrolyse cFDA by action of non‐specific intracellular esterases to produce fluorescent carboxyfluorescein (cF). cF negative charge causes fluorochrome retention within bacterial cells allowing cell enzymatic activity evaluation (Léonard et al. 2016; Barbosa et al. 2020). PEA caused higher sizes of ETEC cell subpopulations (P < 0·001) without enzymatic activity (cF−) than PEC. The exposure of ETEC strains to 62·5 and 31·25 mg ml−1 of PEA led to sizes of cell subpopulations without enzymatic activity ranging from 93·0–96·4% and 87·4–91·3%, respectively (Table 2), while the exposure to 62·5 and 31·25 mg ml−1 of PEC led to sizes of cell subpopulations without enzymatic activity ranging from 65·9–84·4% and 19·4–67·0%, respectively (Table 3). The lowest sizes (P < 0·05) of cell subpopulations without enzymatic activity were found to ETEC ECL 20440 following the exposure to PEC.
The CTC is a membrane‐permeable and non‐fluorescent compound. Dehydrogenase enzymes associated with electron transport system in bacterial cells can reduce CTC to produce a red fluorescent formazan derivative (CTC‐formazan), which indicates respiratory activity and bacterial viability (Créach et al. 2003; Nie et al. 2016; de Sousa Guedes and de Souza 2018). Exposure of ETEC strains to 62·5 and 31·2 mg ml−1 of PEA led to sizes of cell subpopulations without respiratory activity (CTC−) ranging from 61·63–99·30% and 58·07–97·78%, respectively (Table 2). The exposure to 62·5 and 31·2 mg ml−1 of PEC led to sizes of cell subpopulations without respiratory activity ranging from 62·3–83·1% and 32·3–88·1%, respectively (Table 3). The highest sizes (P < 0·001) of cell subpopulations without respiratory activity were found for ETEC ECL21858 regardless of the tested extract.
The extrusion of toxic compounds via efflux pump activity is crucial to keep homeostasis and defense mechanisms in bacterial cells. EB is a membrane‐permeant product able to enter bacterial cell and interact with DNA to produce fluorescence. Functional efflux activity in bacterial cells causes EB pumping out via a non‐specific proton anti‐port transport system (Léonard et al. 2016; Miladi et al. 2016). PEA and PEC caused similar sizes (P = 0·220) of cell subpopulations with compromised efflux pump activity (EB+). The exposure of ETEC strains to 62·5 and 31·2 mg ml−1 of PEA led to sizes of cell subpopulations with compromised efflux pump activity (EB+) ranging from 45·6–72·2% and 27·5–47·6%, respectively (Table 2), while the exposure to 62·5 and 31·2 mg ml−1 of PEC led to sizes of cell subpopulations with compromised efflux pump activity ranging from 36·0–56·8% and 33·7–51·9%, respectively (Table 3). These results showing the capability of PEA and PEC of disturbing the efflux activity in ETEC are important since the active extrusion has been a well‐known mechanism involved in ETEC resistance to clinically relevant antibiotics (Davin‐Regli et al. 2019).
The MFC results have shown the capability of PEA and PEC of impairing distinct physiological functions or cell structures of ETEC, including cell membrane polarization and permeability, as well as enzymatic, respiratory and efflux activities. This is the first study reporting the physiological responses of ETEC to the exposure to fruit by‐product phenolic‐rich extracts. Early studies have suggested the cell membrane as the main target of phenolic compounds in bacterial cells, as well as ion gradient collapse leading to membrane hyper ionization and alterations in fatty acid membrane composition leading to decreased membrane fluidity as mechanisms involved in bacterial cell membrane dysfunctions caused by phenolic compounds (Lacombe et al. 2013; Wang et al. 2018). However, the electronegativity of the outer membrane of Gram‐negative bacteria (e.g., E. coli) could result in lower interaction of phenolic compounds with bacterial cell membrane in comparison with Gram‐positive bacteria (Lima et al. 2019). It could justify the overall lower sizes of ETEC cell subpopulations with damaged cell membrane (PI+) following the exposure to PEA and PEC compared with the sizes of cell subpopulations with damage in other measured physiological functions.
The hydrophobic nature and occurrence of multiple hydroxyl groups in phenolic compounds could mediate the evolution of their impairing effects on bacterial membrane structure and stability, resulting in delayed effective transport, enzymatic function and energy metabolism disruption (Cushnie and Lamb 2005; Duarte et al. 2015; Singh et al. 2016; Aldulaimi 2017). Although it has been reported that bacterial cell subpopulations could still have metabolic activity under stress conditions even being not capable of growing on agar medium to form visible colonies (Léonard et al. 2016; de Sousa Guedes and de Souza 2018; Barbosa et al. 2020), the results of viable counts reductions and physiological function disruptions have shown the remarkable inhibitory effects of PEA and PEC on target ETEC strains. It is noteworthy that 62·5 mg ml−1 of PEA and PEC caused viable count reductions of ≥5 log on target ETEC strains and induced sizes of ETEC cell subpopulations without metabolic/respiratory activity (cF− and CTC−) of >80%, which indicate bacterial death or no cell ability to revert to physiologically active conditions (de Sousa Guedes and de Souza 2018; Barbosa et al. 2020).
In conclusion, the results showed that agro‐industrial by‐products of acerola and cashew could produce phenolic‐rich extracts with inhibitory extracts on distinct ETEC strains. The concentrations of 15·6, 31·2 and 62·5 mg ml−1 of PEA and PEC caused significant reductions in viable counts of target ETEC strains during 24 h of exposure, standing out the ≥3 logs reductions caused by 62·5 mg ml−1 of these extracts. The inhibitory effects of PEA and PEC on target ETEC strains could involve a multi‐target action mode causing impairments in different physiological functions of bacterial cells, including the depolarization and cell membrane permeabilization and enzymatic, respiratory and efflux activities. PEA and PEC could represent novel value‐added antibacterial substances recoverable from agro‐industrial by‐products. These phenolic‐rich extracts should be considered as possible solutions to control ETEC infection in the human and veterinary fields.
Materials and methods
Fruit by‐products
The acerola (Malpighia emarginata L.), cashew apple (A. occidentale L.) and mango (M. indica L.) by‐products were obtained from two different fruit pulp processing companies located in the city of João Pessoa (PB, Brazil; Geographical coordinates: latitude −7·11532, longitude −34·861; 7°6′55″S, 34°51′40″W). Approximately 1 kg of each by‐product from three different fruit processing batches were pooled for a total of 3 kg, frozen (−18 ± 2°C, 24 h) and freeze‐dried (–50 ± 2°C, <130 µHg, 18 h) with a bench‐top lyophilizer (model LI‐101; Liotop, São Carlos, SP, Brazil). The freeze‐dried materials were ground with a domestic blender and stored hermetically with protection from light under freezing (−18 ± 2°C) up to the extract preparation step (Duarte et al. 2017).
Preparation of phenolic‐rich extracts from fruit by‐products
Freeze‐dried fruit by‐products were suspended in 80% ethanol (Neon, São Paulo, SP, Brazil) in a proportion of 1 : 10 and homogenized with an orbital shaker (model TE‐141; Tecnal, Piracicaba, SP, Brazil) at 200 rev min−1 for 2 h. Subsequently, the extracts were centrifuged (4000 g , 10 min, 4°C), supernatants were collected and subjected to total ethanol removal with a rotary evaporator (Model R‐3; Buchi, Valinhos, SP, Brazil) at 45 ± 2°C, frozen (−18 ± 2°C, 24 h) and freeze‐dried (lyophilizer model LI‐101; Liotop, −50 ± 2°C, <130 µHg, 18 h) (Shen et al. 2014; Paz et al. 2015; Singh et al. 2016). The obtained phenolic‐rich extracts from by‐products of acerola (PEA), cashew apple (PEC) and mango (PEM) were tested separately in experiments.
ETEC strains and inoculum preparation
Five distinct ETEC strains were used as target microorganisms, namely ECL 19790 (STb virotype, isolated from faeces of pig (growing‐finishing, 90–240 days) with diarrhoea), ECL 20440 (STb virotype, isolated from ileum of pig (nursery, 22–89 days) with diarrhoea), ECL 23141 (STb virotype, isolated from ileum of pig (nursery, 22–89 days) with diarrhoea), ECL 20898 (STb virotype, isolated from ileum of pig (nursery, 22–89 days) with diarrhoea) and ECL 21858 (STb virotype, isolated from faeces of pig (nursery, 22–89 days) with diarrhoea), which were supplied by the Reference Laboratory for E. coli (Faculty of Veterinary Medicine, Université de Montréal, Québec, Canada). For the preparation of inoculum used in experiments, a loopful from working culture of each strain was resuspended in 5 ml of BHI broth and incubated overnight (37 ± 1°C). Subsequently, the optical density of the culture at 625 nm was adjusted to approximately 0·1, which provided viable counts of 7–8 log colony units per millilitre—CFU per ml, to obtain the standard cell suspensions (McMahon et al. 2008; Almeida et al. 2019). Each strain was tested separately in experiments.
Determination of phenolic compounds in fruit by‐product phenolic‐rich extracts
The PEA, PEC and PEM were diluted (1 : 2, v/v) in 30% ethanol (Modern Chemistry, Barueri, SP, Brazil) and filtered with a 0·45 μm nylon membrane (K18‐430 filter; Kasvi, São José dos Pinhais, PR, Brazil). The identification of the phenolic compounds was done with high‐performance liquid chromatography using an Agilent 1260 Infinity LC system liquid chromatograph (Agilent Technologies, Santa Clara, CA) coupled to a diode arrangement detector (DAD) (model G1315D). A Zorbax Eclipse Plus RP‐C18 column (100 × 4·6 mm, 3·5 µm) and a Zorbax C18 pre‐column (12·6 × 4·6 mm, 5 µm) (Agilent Zorbax, Santa Clara, CA) were used. The other analytical conditions were: temperature 35°C; injection volume 20 μl of samples previously diluted in solvent A and filtered through a 0·45 µm membrane (K18‐430 filter; Kasvi); solvent flow of 0·8 ml min−1; separation gradient from 0 to 5 min: 5% B, 5–14 min: 23% B, 14–30 min: 50% B, 30–33 min 80% B; solvent A was a phosphoric acid solution (0·1 mol l−1, pH 2); and solvent B methanol acidified with 0·5% H3PO4.
The quantification was done by using calibration curves of the external standards (Sigma‐Aldrich, St. Louis, MO) according to a validated method (Padilha et al. 2017). The spectral purity of the peaks was verified using the threshold tool to ensure the identification accuracy compared with the external standards. All determined phenolic compounds had calibration curves with R 2 ≥ 0·998. The detection was done at 280 nm for gallic acid, syringic acid, cis‐resveratrol, hesperidin and naringenin; at 220 nm for epicatechin, catechin, epicatechin‐gallate, epigallocatechin‐gallate, procyanidin A2, procyanidin B1 and procyanidin B2; at 320 nm for trans‐caftaric acid, ρ‐coumaric acid, chlorogenic acid, caffeic acid and trans‐resveratrol; at 360 nm for kaempferol, quercetin 3‐glucoside, rutin and myricetin; and at 520 nm for malvidin 3‐glucoside, cyanidin 3‐glucoside, malvidin 3,5‐diglucoside, cyanidin 3,5‐diglucoside, pelargonidin 3‐glucoside, delphinidin 3‐glucoside, pelargonidin 3,5‐diglucoside and petunidin 3‐glucoside. Data were processed with OpenLAB CDS ChemStation Edition software (Agilent Technologies). Results were expressed in mg 100 g−1 of extract (Padilha et al. 2017).
Determination of minimum inhibitory concentration and minimum bactericidal concentration of fruit by‐products phenolic‐rich extracts
The MIC of PEA, PEC and PEM was determined with broth microdilution. A 50 µl‐aliquot of PEA, PEC and PEM diluted in sterilized BHI to reach concentrations of 4000 mg ml−1 was distributed into wells of the 96‐well microplate containing 50 µl of BHI broth. Subsequently, 50 μl were transferred to the following wells through geometric dilutions and 50 μl aliquot of the tested strain was added to each well (reaching a final viable count of 6–7 log CFU per ml). The final concentrations of phenolic‐rich extracts varied from 0·98 to 1000 mg ml−1. Each microplate included a set of positive (BHI broth with ETEC inoculum) and negative controls (non‐inoculated BHI broth). At the end of the incubation period (37 ± 1°C for 24 h), the MIC was the lowest concentration of each extract where no visual growth of target ETEC strain was observed.
To determinate the MBC, an aliquot (100 µl) of each microplate well was diluted in sterile peptone water (0·1 g peptone 100 ml−1), each dilution (10 µl) was inoculated on BHI agar and incubated (37 ± 1°C, 24 h). The MBC was the lowest concentration of each extract causing reduction of >3 logs CFU per ml of the initial counts of target ETEC strain (LaPlante 2007; CLSI 2019).
Effects of fruit by‐products phenolic‐rich extracts on ETEC viable counts
The effects of PEA and PEC on the viable counts of ETEC strains were evaluated during 24 h. The cell suspension of target ETEC strain (200 µl) was added to BHI broth (2 ml, reaching a final viable count of 6–7 log CFU per ml) with different concentrations of PEA and PEC (15·6, 31·2 and 62·5 mg ml−1), followed by incubation at 37 ± 1°C. On different incubation periods (i.e., zero—immediately after homogenization, 2, 4, 8, 12 and 24 h), an aliquot (100 µl) of the cultivation medium was serially diluted in sterile peptone water (0·1 g peptone 100 ml−1) and each dilution (10 µl) was inoculated on BHI agar. At the end of the incubation period (37 ± 1°C, 24 h), the visible colonies on agar were counted and results were expressed as log CFU per ml. A control consisting of inoculated BHI broth without phenolic‐rich extract was used for each ETEC strain (Almeida et al. 2019; Barbosa et al. 2019). The detection limit of the test was 2 log CFU per ml.
Effects of fruit by‐products phenolic‐rich extracts on ETEC cellular functions
MFC was used to measure the effects of PEA and PEC on distinct physiological functions of the distinct ETEC strains (ECL20440, ECL23141 and ECL21858). An inoculum aliquot (200 µl) of each ETEC strain was added to BHI broth (2 ml) with or without (control) PEA or PEC (31·25 and 62·50 mg ml−1) and maintained at 37 ± 1°C for 24 h. Afterwards, the bacterial cells were harvested (4500 g , 15 min, 4°C), washed and re‐suspended in phosphate buffer solution (PBS, 8 g l−1 of NaCl, 0·2 g l−1 of KCl, 1·44 g l−1 of Na2HPO4 and 0·24 g l−1 of KH2PO4; pH 7·4). The bacterial cell functions were evaluated using PI (Sigma‐Aldrich) to measure membrane integrity, bis‐1,3‐dibutylbarbutiric acid (BOX; Molecular Probes, Invitrogen, Eugene, OR) to measure membrane potential, carboxyfluorescein diacetate (cFDA; ThermoFisher Scientific, Bartleville, OK) to measure enzymatic activity, 5‐cyano‐2,3‐ditolyl tetrazolium chloride (CTC; Polysciences, Warrington, PA) to measure respiratory activity and ethidium bromide (EB; Sigma‐Aldrich) to measure efflux activity (de Sousa Guedes and de Souza 2018; Almeida et al. 2019; Araújo et al. 2020).
For the membrane integrity and efflux activity, the bacterial cells were suspended in PBS with PI (1 µg ml−1) and EB (10 µg ml−1), respectively. For membrane potential and enzymatic activity, the bacterial cells were suspended in PBS with 4 mmol l−1 EDTA containing BOX (2·5 µg ml−1) and cFDA (15 µg ml−1), respectively. For the respiratory activity, bacterial cells were suspended in PBS with glucose 1% (w/v) and CTC (5 mmol l−1). Staining was done by incubation at 37 ± 1°C for 15 min except for CTC where the cells were incubated for 30 min (Wang et al. 2010; Silva et al. 2011; de Sousa Guedes and de Souza 2018).
A BD Accuri C6 flow cytometer (BD Accuri C6; Becton Dickson, Franklin Lakes, NJ) with 488 nm excitation from a blue solid‐state laser was used to measure the sizes of distinct bacterial cell subpopulations. Green fluorescence was collected in FL1 channel (533 ± 30 nm) and red fluorescence in FL3 channel (>670 nm). Fluorescence signals (pulse area measurements) were collected by FL1 (cFDA and BOX) and FL3 (PI, EB and CTC) bandpass filters. Threshold point was adjusted for FSC (12 000) to eliminate noise or particles (cellular debris) much smaller than intact cells. Sample acquisition was operated at 2500 events per second, and a total of 10 000 events were acquired for each sample. Cytometry data analysis was done with BD Accuri C6 Software (de Sousa Guedes and de Souza 2018; Barbosa et al. 2020).
Statistical analysis
The experiments were done in triplicate in three independent tests, and results were expressed as average ± standard deviation. Results of determination of MIC and MBC are expressed as modal values. Kolmogorov–Smirnov normality test was run to assess whether data had normal distribution. The inferential statistical analysis was done with Kruskal–Wallis test, Friedman test or Mann–Whitney U test considering a P value of <0·05 for significance. A Spearman’s correlation analysis was run to determine the coefficient of correlation between continuous variables. Statistical analysis was done with software Jamovi (ver. 1.6; Computer Software, retrieved from https://www.jamovi.org).
Author’s contribution
Conceptualization: MCL, CPS, JDD, ELS. Data curation: MCL, ELS; Formal analysis: MCL, CPS, JDD, ELS; Funding acquisition: ELS; Investigation: MCL, ELS; Methodology: MCL, MM, MSL, ELS; Project administration: ELS; Resources: MCL, MSL, ELS; Supervision: ELS; Visualization; Writing – original draft: MCL, MM, CPS, JDD, ELS; Writing – review and editing: MCL, JDD, ELS.
Conflict of Interest
No conflict of interest has been declared.
Acknowledgement
Authors thank CAPES – Brazil (Finance code 001) and FAPESP (Sao Paulo – Brazil, Grant 2016/13423‐5) for funding partially this research, FAPESQ (Paraíba, Brazil) for a PhD scholarship awarded to M. C. Lima and Université of Montréal (Canada) for providing the ETEC strains.
References
- de Albuquerque, M.A.C. , Levit, R. , Beres, C. , Bedani, R. , de Moreno de LeBlanc, A. , Saad, S.M.I. and LeBlanc, J.G. (2019) Tropical fruit by‐products water extracts of tropical fruit by‐products as sources of soluble fibres and phenolic compounds with potential antioxidant, anti‐inflammatory, and functional properties. J Funct Foods 52, 724–733. [Google Scholar]
- Aldulaimi, O.A. (2017) General overview of phenolics from plant to laboratory, good antibacterials or not. Pharmacogn Rev 11, 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alejo‐Armijo, A. , Glibota, N. , Frías, M.P. , Altarejos, J. , Gálvez, A. , Ortega‐Morente, E. and Salido, S. (2017) Antimicrobial and antibiofilm activities of procyanidins extracted from laurel wood against a selection of foodborne microorganisms. Int J Food Sci Technol 52, 679–686. [Google Scholar]
- Almeida, E.T.D.C. , de Souza, G.T. , de Sousa Guedes, J.P. , Barbosa, I.M. , de Sousa, C.P. , Castellano, L.R.C. , Magnani, M. and de Souza, E.L. (2019) Mentha piperita L. essential oil inactivates spoilage yeasts in fruit juices through the perturbation of different physiological functions in yeast cells. Food Microbiol 82, 20–29. [DOI] [PubMed] [Google Scholar]
- Araújo, C.M. , Sampaio, K.B. , Menezes, F.N.D.D. , Almeida, E.T.D.C. , Lima, M.D.S. , Viera, V.B. , Garcia, E.F. , Gómez‐Zavaglia, A. et al. (2020) Protective effects of tropical fruit processing coproducts on probiotic Lactobacillus strains during freeze‐drying and storage. Microorganisms 8, 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif, A. , Farooq, U. , Akram, K. , Hayat, Z. , Shafi, A. , Sarfraz, F. , Sidhu, M.A.I. , Rehman, H. et al. (2016) Therapeutic potentials of bioactive compounds from mango fruit wastes. Trends Food Sci Technol 53, 102–112. [Google Scholar]
- Bag, A. and Chattopadhyay, R.R. (2017) Synergistic antibacterial and antibiofilm efficacy of nisin in combination with p‐coumaric acid against food‐borne bacteria Bacillus cereus and Salmonella typhimurium . Lett Appl Microbiol 65, 366–372. [DOI] [PubMed] [Google Scholar]
- Barbosa, I.M. , da Cruz Almeida, E.T. , Castellano, L.R.C. and de Souza, E.L. (2019) Influence of stressing conditions caused by organic acids and salts on tolerance of Listeria monocytogenes to Origanum vulgare L. and Rosmarinus officinalis L. essential oils and damage in bacterial physiological functions. Food Microbiol 84, 103240. [DOI] [PubMed] [Google Scholar]
- Barbosa, I.M. , da Cruz Almeida, É.T. , Gomes, A.C.A. and de Souza, E.L. (2020) Evidence on the induction of viable but non‐culturable state in Listeria monocytogenes by Origanum vulgare L. and Rosmarinus officinalis L. essential oils in a meat‐based broth. Innov Food Sci Emerg Technol 62, 102351. [Google Scholar]
- Batista, K.S. , Alves, A.F. , Lima, M.D.S. , da Silva, L.A. , Lins, P.P. , de Sousa Gomes, J.A. , Silva, A.S. , Toscano, L.T. et al. (2018) Beneficial effects of consumption of acerola, cashew or guava processing by‐products on intestinal health and lipid metabolism in dyslipidaemic female Wistar rats. Br J Nutr 119, 30–41. [DOI] [PubMed] [Google Scholar]
- Belwal, T. , Devkota, H.P. , Hassan, H.A. , Ahluwalia, S. , Ramadan, M.F. , Mocan, A. and Atanasov, A.G. (2018) Phytopharmacology of Acerola (Malpighia spp.) and its potential as functional food. Trends Food Sci Technol 74, 99–106. [Google Scholar]
- CLSI ‐ Clinical and Laboratory Standards Institute (2019) Performance Standards for Antimicrobial Susceptibility Testing 29th edn. CLSI standard M100. Wayne, PA: CLSI. [Google Scholar]
- Coelho, E.M. , de Souza, M.E.A.O. , Corrêa, L.C. , Viana, A.C. , de Azevêdo, L.C. and Lima, M.D.S. (2019) Bioactive compounds and antioxidant activity of mango peel liqueurs (Mangifera indica L.) produced by different methods of maceration. Antioxidants 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coman, M.M. , Oancea, A.M. , Verdenelli, M.C. , Cecchini, C. , Bahrim, G.E. , Orpianesi, C. , Cresci, A. and Silvi, S. (2018) Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur Food Res Technol 244, 735–745. [Google Scholar]
- Créach, V. , Baudoux, A.C. , Bertru, G. and Rouzic, B.L. (2003) Direct estimate of active bacteria: CTC use and limitations. J Microbiol Methods 52, 19–28. [DOI] [PubMed] [Google Scholar]
- Cushnie, T.P.T. and Lamb, A.J. (2005) Antimicrobial activity of flavonoids. Int J Antimicrob Agents 26, 343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davin‐Regli, A. , Guerin‐Faublée, V. and Pagès, J.M. (2019) Modification of outer membrane permeability and alteration of LPS in veterinary enterotoxigenic Escherichia coli . Res Vet Sci 124, 321–327. [DOI] [PubMed] [Google Scholar]
- Díaz‐Gómez, R. , Toledo‐Araya, H. , López‐Solís, R. and Obreque‐Slier, E. (2014) Combined effect of gallic acid and catechin against Escherichia coli . LWT ‐ Food Sci Technol 59, 896–900. [Google Scholar]
- Diniz‐Silva, H.T. , Magnani, M. , de Siqueira, S. , de Souza, E.L. and de Siqueira‐Júnior, J.P. (2017) Fruit flavonoids as modulators of norfloxacin resistance in Staphylococcus aureus that overexpresses norA. LWT ‐ Food Sci Technol 85, 324–326. [Google Scholar]
- Duarte, A. , Alves, A.C. , Ferreira, S. , Silva, F. and Domingues, F.C. (2015) Resveratrol inclusion complexes: antibacterial and anti‐biofilm activity against Campylobacter spp. and Arcobacter butzleri . Food Res Int 77, 244–250. [Google Scholar]
- Duarte, F.N.D. , Rodrigues, J.B. , Lima, M.C. , Lima, M.S. , Pacheco, M.T.B. , Pintado, M.M.E. , de Souza Aquino, J. and de Souza, E.L. (2017) Potential prebiotic properties of cashew apple (Anacardium occidentale L.) agro‐industrial byproduct on Lactobacillus species . J Sci Food Agric 97, 3712–3719. [DOI] [PubMed] [Google Scholar]
- Dubreuil, J.D. (2020) Fruit extracts to control pathogenic Escherichia coli: a sweet solution. Heliyon 6, e03410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubreuil, J.D. , Isaacson, R.E. and Schifferli, D.M. (2016) Animal enterotoxigenic Escherichia coli . EcoSal Plus 7, ESP‐0006‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierascu, R.C. , Sieniawska, E. , Ortan, A. , Fierascu, I. and Xiao, J. (2020) Fruits by‐products – a source of valuable active principles. A short review. Front Bioeng Biotechnol 8, 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, A.C.A. , da Costa Lima, M. , de Oliveira, K.Á.R. , dos Santos Lima, M. , Magnani, M. , Câmara, M.P.S. and de Souza, E.L. (2020) Coatings with chitosan and phenolic‐rich extract from acerola (Malpighia emarginata D.C.) or jabuticaba (Plinia jaboticaba (Vell.) Berg) processing by‐product to control rot caused by Lasiodiplodia spp. in papaya (Carica papaya L.) fruit. Int J Food Microbiol 331, 108694. [DOI] [PubMed] [Google Scholar]
- Gomes, T.A.T. , Elias, W.P. , Scaletsky, I.C.A. , Guth, B.E.C. , Rodrigues, J.F. , Piazza, R.M.F. , Ferreira, L.C.S. , Martinez, M.B. et al. (2016) Diarrheagenic Escherichia coli . Braz J Microbiol 47, 3–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, A. , Friedrich, M. , da Matta, V.M. , Herbster Moura, C.F. and Marx, F. (2012) Changes in phenolic composition, ascorbic acid and antioxidant capacity in cashew apple (Anacardium occidentale L.) during ripening. Fruits 67, 267–276. [Google Scholar]
- Gullon, B. , Pintado, M.E. , Pérez‐Álvarez, J.A. and Viuda‐Martos, M. (2016) Assessment of polyphenolic profile and antibacterial activity of pomegranate peel (Punica granatum) flour obtained from co‐product of juice extraction. Food Cont 59, 94–98. [Google Scholar]
- Haminiuk, C.W.I. , Maciel, G.M. , Plata‐Oviedo, M.S.V. and Peralta, R.M. (2012) Phenolic compounds in fruits ‐ an overview. Int J Food Sci Technol 47, 2023–2044. [Google Scholar]
- Jang, J. , Hur, H.G. , Sadowsky, M.J. , Byappanahalli, M.N. , Yan, T. and Ishii, S. (2017) Environmental Escherichia coli: ecology and public health implications ‐ a review. J Appl Microbiol 47, 2023–2044. [DOI] [PubMed] [Google Scholar]
- Khalifa, I. , Zhu, W. , Li, K. and Li, C. (2018) Polyphenols of mulberry fruits as multifaceted compounds: compositions, metabolism, health benefits, and stability ‐ a structural review. J Funct Foods 40, 28–43. [Google Scholar]
- Khalil, I.A. , Troeger, C. , Blacker, B.F. , Rao, P.C. , Brown, A. , Atherly, D.E. , Brewer, T.G. , Engmann, C.M. et al. (2018) Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis 18, 1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe, A. , McGivney, C. , Tadepalli, S. , Sun, X. and Wu, V.C.H. (2013) The effect of American cranberry (Vaccinium macrocarpon) constituents on the growth inhibition, membrane integrity, and injury of Escherichia coli O157:H7 and Listeria monocytogenes in comparison to Lactobacillus rhamnosus . Food Microbiol 34, 352–359. [DOI] [PubMed] [Google Scholar]
- LaPlante, K.L. (2007) In vitro activity of lysostaphin, mupirocin, and tea tree oil against clinical methicillin‐resistant Staphylococcus aureus . Diagn Microbiol Infect Dis 57, 413–418. [DOI] [PubMed] [Google Scholar]
- Léonard, L. , Chibane, L.B. , Bouhedda, B.O. , Degraeve, P. and Oulahal, N. (2016) Recent advances on multi‐parameter flow cytometry to characterize antimicrobial treatments. Front Microbiol 7, 1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima, M.C. , Paiva de Sousa, C. , Fernandez‐Prada, C. , Harel, J. , Dubreuil, J.D. and de Souza, E.L. (2019) A review of the current evidence of fruit phenolic compounds as potential antimicrobials against pathogenic bacteria. Microb Pathog 130, 259–270. [DOI] [PubMed] [Google Scholar]
- Lima, M.C. and de Souza, E.L. (2020) A systematic quantitative analysis of the published literature on the efficacy of essential oils as sanitizers in fresh leafy vegetables. Crit Rev Food Sci Nutr 10, 1–14. [DOI] [PubMed] [Google Scholar]
- Luppi, A. (2017) Swine enteric colibacillosis: diagnosis, therapy and antimicrobial resistance. Porc Heal Manag 3, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon, M.A.S. , Tunney, M.M. , Moore, J.E. , Blair, I.S. , Gilpin, D.F. and McDowell, D.A. (2008) Changes in antibiotic susceptibility in staphylococci habituated to sub‐lethal concentrations of tea tree oil (Melaleuca alternifolia). Lett Appl Microbiol 47, 263–268. [DOI] [PubMed] [Google Scholar]
- Miladi, H. , Zmantar, T. , Chaabouni, Y. , Fedhila, K. , Bakhrouf, A. , Mahdouani, K. and Chaieb, K. (2016) Antibacterial and efflux pump inhibitors of thymol and carvacrol against food‐borne pathogens. Microb Pathog 99, 95–100. [DOI] [PubMed] [Google Scholar]
- Nie, X. , Liu, W. , Chen, M. , Liu, M. and Ao, L. (2016) Flow cytometric assessment of the effects of chlorine, chloramine, and UV on bacteria by using nucleic acid stains and 5‐cyano‐2,3‐ditolyltetrazolium chloride. Front Environ Sci Eng 10, 1–12. [Google Scholar]
- Ojha, D. and Patil, K.N. (2019) p‐Coumaric acid inhibits the Listeria monocytogenes RecA protein functions and SOS response: an antimicrobial target. Biochem Biophys Res Commun 517, 655–661. [DOI] [PubMed] [Google Scholar]
- Padilha, C.V.D.S. , Miskinis, G.A. , de Souza, M.E.A.O. , Pereira, G.E. , de Oliveira, D. , Bordignon‐Luiz, M.T. and Lima, M.D.S. (2017) Rapid determination of flavonoids and phenolic acids in grape juices and wines by RP‐HPLC/DAD: method validation and characterization of commercial products of the new Brazilian varieties of grape. Food Chem 228, 106–115. [DOI] [PubMed] [Google Scholar]
- Paz, M. , Gúllon, P. , Barroso, M.F. , Carvalho, A.P. , Domingues, V.F. , Gomes, A.M. , Becker, H. , Longhinotti, E. et al. (2015) Brazilian fruit pulps as functional foods and additives: evaluation of bioactive compounds. Food Chem 172, 462–468. [DOI] [PubMed] [Google Scholar]
- Rezaeinejad, S. and Ivanov, V. (2011) Heterogeneity of Escherichia coli population by respiratory activity and membrane potential of cells during growth and long‐term starvation. Microbiol Res 166, 129–135. [DOI] [PubMed] [Google Scholar]
- Sanhueza, L. , Melo, R. , Montero, R. , Maisey, K. , Mendoza, L. and Wilkens, M. (2017) Synergistic interactions between phenolic compounds identified in grape pomace extract with antibiotics of different classes against Staphylococcus aureus and Escherichia coli . PLoS One 12, e0172273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, X. , Sun, X. , Xie, Q. , Liu, H. , Zhao, Y. , Pan, Y. , Hwang, C.A. and Wu, V.C.H. (2014) Antimicrobial effect of blueberry (Vaccinium corymbosum L.) extracts against the growth of Listeria monocytogenes and Salmonella Enteritidis . Food Cont 35, 159–165. [Google Scholar]
- Silva, F. , Ferreira, S. , Queiroz, J.A. and Domingues, F.C. (2011) Coriander (Coriandrum sativum L.) essential oil: its antibacterial activity and mode of action evaluated by flow cytometry. J Med Microbiol 60, 1479–1486. [DOI] [PubMed] [Google Scholar]
- da Silva, L.M.R. , de Figueiredo, E.A.T. , Ricardo, N.M.P.S. , Vieira, I.G.P. , de Figueiredo, R.W. , Brasil, I.M. and Gomes, C.L. (2014) Quantification of bioactive compounds in pulps and by‐products of tropical fruits from Brazil. Food Chem 143, 398–404. [DOI] [PubMed] [Google Scholar]
- Singh, J.P. , Kaur, A. , Singh, N. , Nim, L. , Shevkani, K. , Kaur, H. and Arora, D.S. (2016) In vitro antioxidant and antimicrobial properties of jambolan (Syzygium cumini) fruit polyphenols. LWT ‐ Food Sci Technol 65, 1025–1030. [Google Scholar]
- Sinsinwar, S. and Vadivel, V. (2020) Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS‐mediated oxidative stress. Appl Microbiol Biotechnol 104, 8279–8297. [DOI] [PubMed] [Google Scholar]
- de Sousa Guedes, J.P. and de Souza, E.L. (2018) Investigation of damage to Escherichia coli, Listeria monocytogenes and Salmonella Enteritidis exposed to Mentha arvensis L. and M. piperita L. essential oils in pineapple and mango juice by flow cytometry. Food Microbiol 76, 564–571. [DOI] [PubMed] [Google Scholar]
- de Souza, E.L. (2016) The effects of sublethal doses of essential oils and their constituents on antimicrobial susceptibility and antibiotic resistance among food‐related bacteria: a review. Trends Food Sci Technol 56, 1–12. [Google Scholar]
- van Vuuren, S.F. (2008) Antimicrobial activity of South African medicinal plants. J Ethnopharmacol 119, 462–472. [DOI] [PubMed] [Google Scholar]
- Wang, L.‐H. , Zeng, X.‐A. , Wang, M.‐S. , Brennan, C.S. and Gong, D. (2018) Modification of membrane properties and fatty acids biosynthesis‐related genes in Escherichia coli and Staphylococcus aureus: Implications for the antibacterial mechanism of naringenin. Biochim Biophys Acta ‐ Biomembr 1860, 481–490. [DOI] [PubMed] [Google Scholar]
- Wang, Y. , Claeys, L. , Van Der Ha, D. , Verstraete, W. and Boon, N. (2010) Effects of chemically and electrochemically dosed chlorine on Escherichia coli and Legionella beliardensis assessed by flow cytometry. Appl Microbiol Biotechnol 87, 331–341. [DOI] [PubMed] [Google Scholar]
- Wilkinson, M.G. (2018) Flow cytometry as a potential method of measuring bacterial viability in probiotic products: a review. Trends Food Sci Technol 78, 1–10. [Google Scholar]


