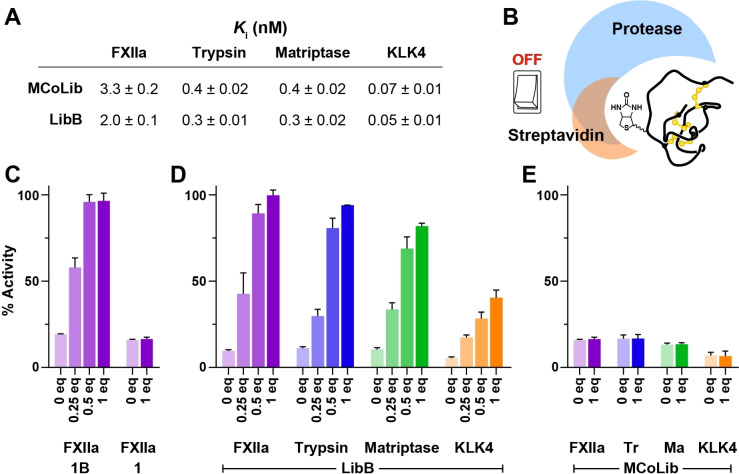
Figure 5.
Switching OFF biotin‐labelled knottins using an external effector. A) K i values (±standard error) for the biotin‐labelled inhibitor LibB compared with its non‐labelled counterpart MCoLib. Data are from three experiments performed in triplicate. B) Schematic illustrating the concept of an affinity tag OFF switch based on knottin labelling at P4′ that introduces overlapping binding sites for streptavidin and the target protease. C) Recovery of enzyme activity (y‐axis) after adding 0.25–1 equiv streptavidin (x‐axis) to FXIIa incubated with 1B (≈1 : 20 ratio FXIIa:1B). Activity data are expressed as a % relative to controls with FXIIa and substrate only (mean±SD from three experiments). No recovery of activity was observed for the unlabelled inhibitor 1. D) Recovery of enzyme activity after adding streptavidin to FXIIa (purple), trypsin (blue), matriptase (green) or KLK4 (orange) incubated with LibB. E) No recovery of activity was observed for the non‐labelled inhibitor MCoLib.