Abstract
Background and Aims
Although fecal microbiota transplantation (FMT) from healthy donors has been shown to have hypoglycemic effects in animal models of diabetes, its clinical impact in patients with abnormal blood glucose metabolism is unclear, especially in southern Chinese populations. The aim of this study was to investigate the feasibility and efficacy of washed microbiota transplantation (WMT) in the treatment of abnormal blood glucose metabolism in a population in southern China.
Methods
The clinical data of patients with different indications who received 1-3 treatments of WMT were retrospectively collected. The changes of blood glucose, blood lipids, blood pressure, liver function and blood routine before and after WMT were compared, such as fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), triglyceride (TG), systolic blood pressure (SBP), white blood cells (WBC), lymphocytes (LY) and platelets (PLT), etc.
Results
A total of 195 patients were included in the First Affiliated Hospital of Guangdong Pharmaceutical University, including 20 patients with high blood glucose and 175 patients with normal blood glucose. WMT has a significant effect in reducing short term blood glucose level (FBG) in patients with high blood glucose (p < 0.05). The fasting blood glucose (FBG) of 72.22% of patients with high blood glucose decreased to normal in a short term (about 1 month) (p < 0.001); In the medium term (about 2 months), there was a significant hypolipidemic (TG) (p = 0.043) effect, long term (about 6 months) significant blood pressure lowering (SBP, p = 0.048) effect. Overall, WMT significantly reduced the risk of high risk classes of Atherosclerotic Cardiovascular Disease (ASCVD) in the short term (p = 0.029) and medium term (p = 0.050).
Conclusion
WMT can significantly improve blood glucose in patients with high blood glucose, and there is no long-term elevated risk of blood glucose and ASCVD. FBG levels were significantly reduced in both the short and medium term in patients with high blood glucose treated with WMT. Therefore, the regulation of gut microbiota by WMT may provide a new clinical approach for the treatment of abnormal blood glucose metabolism.
Keywords: fecal microbiota transplantation, washed microbiota transplantation (WMT), abnormal blood glucose metabolism, high blood glucose, fasting blood glucose
Introduction
Diabetes mellitus, a chronic disease characterized by relative or absolute insulin deficiency leading to hyperglycemia, is one of the largest global public health problems. The prevalence of diabetes has continued to increase in most developed and developing countries in recent decades (1, 2). To date, the International Diabetes Federation (IDF) estimates that approximately 451 million adults (aged 18-99) were living with diabetes worldwide in 2017, and this is expected to increase to 693 million by 2045 if effective prevention methods are not taken (3). In China, the prevalence of diabetes is still rising, with the prevalence increased rapidly to 10.9% - 12.8% from 2010 to 2018. Diabetes awareness (36.5%), treatment (32.2%) and control (49.2%) rates have improved but remain low (4). The prevalence of diabetes was significantly higher in urban areas than in rural areas (12.0% vs 8.9%), and higher in men than in women (11.1% vs 9.6%) (5, 6). Type 2 diabetes mellitus (T2DM) accounts for more than 90% of the diabetic population, and the prevalence of diabetes in the elderly over 60 years old is close to or exceeds 20% (7). At the same time, diabetes is one of the top ten causes of death in the world, and people with diabetes mainly die of diabetes-related complications rather than diabetes itself (8). A large number of these premature deaths can potentially be prevented through prevention or early detection, and improved management of diabetes and these complications.
T2DM patients often have one or more components of metabolic syndrome, such as hypertension, dyslipidemia, obesity, etc., which significantly increase the risk, progression speed and harm of T2DM complications. Therefore, a scientific and reasonable T2DM treatment strategy should be comprehensive, including the control of blood glucose, blood pressure, blood lipids, and body weight, antiplatelet therapy and lifestyle improvement measures. These treatment strategies are mainly lifestyle intervention and drug therapy. A variety of drugs are used to treat T2DM and can be divided into five main categories: drugs that stimulate insulin production by beta cells (sulfonylureas), improve insulin action (thiazolidinediones), delay carbohydrate uptake in the gut (alpha-glucosidase inhibitors), reduce hepatic glucose production (metformin) or target the glucagon-like peptide (GLP-1) axis (GLP-1 receptor agonists or DPP-4 inhibitors) (9). However, long term use of drug therapy can have significant side effects. Therefore, it is of great significance to comprehensively analyze the related factors of diabetes and find a treatment method with less side effects.
Gut microbiota has emerged as an important factor associated with human disease (10, 11). Numerous studies have demonstrated changes in the gut microbiota of T2DM patients by comparing the gut microbiota of T2DM patients with healthy individuals (12, 13). Dietary fiber selectively promotes gut bacteria to alleviate T2DM (14), suggesting that gut microbiota plays an important role in T2DM. In animal models of T2DM, Fecal microbiota transplantation (FMT) can improve HOMA-IR and insulin sensitivity (15), and repair damaged pancreatic islets (16), providing a potential strategy for the treatment of T2DM (17). FMT is a new treatment approach that uses healthy microbial profiles to replace the patient’s own microbiota (18). FMT is of increasing interest (19) and have been successfully used to treat a variety of human diseases, such as inflammatory bowel disease (20), obesity (21), as well as metabolic syndrome (22) and functional bowel disease (23, 24). Whether FMT can improve abnormal blood glucose metabolism is a topic to be explored in clinical medicine.
Washed microbiota transplantation (WMT) is safe, quality-controlled (25), and effective (26) for the treatment of gut microbiota disorders. We attempted to investigate whether WMT could improve blood glucose metabolism in patients undergoing WMT. We hypothesized that WMT could safely and consistently affect patients across a variety of indications, improving abnormal blood glucose metabolism without side effects. Therefore, we conducted a retrospective trial to collect medical data from patients with abnormal blood glucose metabolism treated with WMT.
Materials and methods
Patients
This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China according to the Declaration of Helsinki (no. 2017-98). Written informed consent was obtained and reviewed from all patients. This study included patients who completed 2-4 courses of WMT in our hospital from December 7, 2016 to April 30, 2022. Inclusion criteria: older than 18 years, informed consent, and those who received WMT. Exclusion criteria: pregnant women, patients taking antibiotics, hormones and probiotics during the first 3 months of WMT and during transplantation, and patients taking hypoglycemic drugs after WMT. In the end, a total of 195 people met the requirements.
WMT procedure
The procedure of WMT was in accordance with the Nanjing Consensus on the Methodology of Washed Microbiota Transplantation (26). All healthy fecal donors between the ages of 18 and 25 undergo rigorous consultation, psychological and physical examination, biochemical testing and infectious disease screening. To prepare the washed microbiota, each 100 g of feces and 500 mL of 0.9% saline was used to prepare a homogeneous fecal suspension. The fecal suspension was then subjected to microfiltration (to remove fecal particles, parasite eggs, and fungi) using a smart microbial separation system (GenFMTer; FMT Medical, Nanjing, China). After microfiltration, the fecal supernatant was centrifuged at 1100 × g for 3 min at room temperature. Then, the supernatant obtained after centrifugation was discarded. The microbiota pellet was resuspended in saline, then the microbiota pellet was centrifuged and resuspended 3 times. In the final resuspension, 100 mL of saline was added to the microbiota pellet obtained from 100 g of feces (25). Then, according to each patient’s physical condition and wishes, the fecal suspension was injected into the patient through a nasojejunal tube (upper gastrointestinal tract) or a colonic transendoscopic enteral tube (lower gastrointestinal tract). A WMT course of 3 days, once a day, once a 120ml (27). The results of blood tests and other tests before the first course of treatment are the baseline values, and relevant indicators will be obtained before each subsequent course of treatment. According to the standard treatment time of WMT, short term: about 1 month interval from the first course of WMT; medium term: about 2 months interval from the first course of WMT; long term: about 6 months interval from the first course of WMT. All patients received at least 2 courses of WMT and completed follow-up.
Data collection
The medical records of the patients before treatment (baseline value), short term efficacy, medium term efficacy, and long-term efficacy were collected. Data included age, sex, body mass index (BMI), blood glucose at admission, blood pressure, disease or indication for WMT, and laboratory test results. Blood glucose indicators, namely fasting blood glucose (FBG), glycosylated hemoglobin (HbA1c). Insulin indicators, namely fasting insulin (FI), homeostasis model assessment of insulin resistance (HOMA-IR). Blood lipid indicators, namely total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-c), high density lipoprotein cholesterol (HDL-c), apolipoprotein B (ApoB), non-high density lipoprotein (non-HDL-c), lipoprotein (LIP). Blood pressure indicators, namely systolic blood pressure (SBP) on admission, diastolic blood pressure (DBP) on admission. Liver function indicators, namely alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (ALB), total protein (TP), Albumin/globulin ratio (A/G), leucine aminopeptidase (LAP), glutamyl transpeptidase (GT), glutathione reductase (GR), Direct bilirubin (DBIL), indirect bilirubin (IBIL), total bilirubin (TBIL). Blood routine, that is, red blood cell count (RBC), hemoglobin concentration (HGB), white blood cell count (WBC), lymphocyte count (LY), platelet count (PLT), etc. HOMA-IR values were calculated as previously described (28). According to the Chinese Guidelines for the Prevention and Treatment of Type 2 Diabetes (2020 Edition), patients with high blood glucose were diagnosed (4), and all eligible patients were divided into 2 groups: high blood glucose group, 6.1mmol/L≤FBG or 6.1%<HbA1c and no hypoglycemic drugs, or FBG ≥7.0mmol/L was diagnosed as diabetic but without hypoglycemic drugs. Diagnosed with diabetes and the use of hypoglycemic drugs were excluded. Normal blood glucose group, 3.9mmol/L≤FBG<6.1mmol/L. Hospital-defined hyperlipidemia (TC ≥ 6.2 mmol/L or TG ≥ 2.3 mmol/L or LDL-c ≥ 4.1 mmol/L), hypolipidemia (TC < 3.6 mmol/L or TG < 0.33 mmol/L or LDL-c < 2.07 mmol/L or HDL < 0.91 mmol/L). Hypertension was defined as SBP ≥140 mmHg and/or DBP ≥90 mmHg (29). With reference to the Chinese cardiovascular disease prevention guidelines (2017 edition) (30), ASCVD risk stratification was performed according to baseline and blood lipid status: Those who meet one of the following conditions were directly classified as high-risk groups (1): Diabetes (age ≥ 40 years old) (2). Individuals with extremely high levels of a single risk factor, including: ① LDL-c ≥ 4.9 mmol/L (190 mg/dl) or TC ≥ 7.2 mmol/L (280 mg/dl); ② Grade 3 hypertension; ③ Heavy smoking (≥ 30 sticks/d). According to the 10-year average risk of ASCVD in different combinations, it was defined as low risk, intermediate risk and high risk according to < 5%, 5%-9% and ≥ 10%, respectively. Obese (≥ 28.0), overweight (24.0-28.0), normal weight (18.5-24.0), and underweight (< 18.5) were defined according to BMI (kg/m2) (31). Definition of serious adverse events (AEs): increased frequency of defecation, fever, abdominal pain, flatulence, hematochezia, vomiturition, bloating and herpes zoster, etc (25). All patients were divided into 2 groups according to the above definitions. After all patients received 1-3 WMT treatments and completed follow-up, statistical analysis and evaluation of blood glucose, blood lipids, blood pressure, liver function and blood routine results were performed.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA) and Prism 8 (GraphPad, San Diego, CA, USA). Results are expressed as frequencies and percentages for categorical variables, mean and standard deviation for continuous variables with a normal distribution. Categorical variables were analyzed using chi-square or Fisher’s exact test. For comparison of continuous variables between two independent groups, an unpaired Student’s-t test (normally distributed variables) can be used. Paired data were compared using paired Student’s-t test (normally distributed variables). Two-tailed p-values ≤ 0.05 were considered statistically significant.
Results
Clinical characteristics of patients who underwent WMT
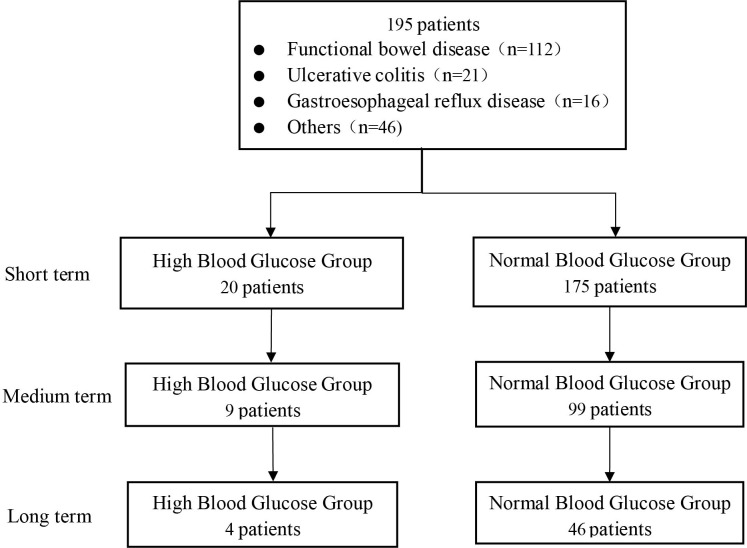
WMT was completed in the First Affiliated Hospital of Guangdong Pharmaceutical University from December 2016 to April 2022. A total of 195 patients met the inclusion criteria (20 in the high blood glucose group and 175 in the normal blood glucose group). Among them, 98 (50.26%) were male and 97 (49.74%) were female, with an average age of 52.59 ± 14.36 years ( Figure 1 ). Table 1 shows the main six disease characteristics of patients undergoing WMT, which are functional bowel disease (n=112, 57.44%, including irritable bowel syndrome, functional constipation), ulcerative colitis (n=21, 10.77%), gastroesophageal reflux disease (n=16, 8.21%), gouty arthritis (n=7, 3.59%), atopic dermatitis (n=6, 3.08%), chemotherapy-related diarrhea (n=6, 3.08%), hyperlipidemia (n=6, 3.08%). Due to the different compliance of patients, WMT treatment may not be completed on schedule. In this study, the time interval of WMT in the enrolled patients was counted, and the number of days was expressed as the median (25%-75%). The blood test results of the patients before the first course of treatment were the baseline values, and the interval between 3 treatments was 35 days (32-42 days) in the short term, 77 days (67-97.75 days) in the medium term, and 183 days (145-202.25 days) in the long term.
Figure 1.
Flow chart of this study.
Table 1.
The main diagnoses of patients receiving washed microbiota transplantation.
| Primary cause of WMT | Number (n) | Percentage (%) |
|---|---|---|
| Functional bowel disease | 112 | 57.44% |
| Ulcerative colitis | 21 | 10.77% |
| Gastroesophageal reflux disease | 16 | 8.21% |
| Gouty arthritis | 7 | 3.59% |
| Atopic dermatitis | 6 | 3.08% |
| Chemotherapy-associated diarrhea | 6 | 3.08% |
| Hyperlipidemia | 6 | 3.08% |
| Radiation enteritis | 5 | 2.56% |
| Hyperuricemia | 3 | 1.54% |
| Hepatitis B cirrhosis | 1 | 0.51% |
| Hyperlipidemic pancreatitis | 1 | 0.51% |
| Functional dysphagia | 1 | 0.51% |
| Senile tremor | 1 | 0.51% |
| Epilepsy | 1 | 0.51% |
| Duodenal stasis | 1 | 0.51% |
| Crohn’s disease | 1 | 0.51% |
| Chronic urticaria | 1 | 0.51% |
| Depression | 1 | 0.51% |
| Parkinson’s syndrome | 1 | 0.51% |
| Bipolar disorder | 1 | 0.51% |
| Psoriasis vulgaris | 1 | 0.51% |
| Perianal eczema | 1 | 0.51% |
| Total | 195 | 100% |
The demographic and clinical characteristics of high blood glucose group versus normal blood glucose group patients are compared in Table 2 . Due to different compliance, not all patients have complete data, so the number of patients in each group is different for each indicator. There was no significant difference in age, gender ratio, and BMI between high blood glucose group and normal blood glucose group, indicating that the basic conditions of the study population were not significantly different, reducing the confounding factors of this study. The mean FBG of the high blood glucose group was 6.6 ± 1.67mmol/L higher than the definition in Materials and methods, and the mean FBG of the normal blood glucose group was 4.62 ± 0.48mmol/L. There was a significant difference in FBG between the two groups. The average TG in the high blood glucose group was 3.33 ± 4.75mmol/L, which was higher than the definition of hyperlipidemia, and that in the normal blood glucose group was 1.2 ± 0.78mmol/L. There was a significant difference in TG between the two groups. This indicated that the high blood glucose group was accompanied by symptoms of hyperlipidemia in addition to the higher FBG. HbA1c (6.02 ± 0.78 vs 5.41 ± 0.30%), FI (14.71 ± 10.86 vs 8.12 ± 4.93 μU/mL), HOMA-IR (4.26 ± 3.3 vs 1.7 ± 1.1), non-HDL-c (4.15 ± 1.65 vs 3.4 ± 0.98 mmol/L), AST (26.1 ± 11.53 vs 20.98 ± 7.12 U/L), LAP (33.2 ± 8.93 vs 29.71 ± 4.8 U/L), GT (60.34 ± 80.04 vs 23.88 ± 14.04U/L), DBIL (5.82 ± 10.41 vs 4.25 ± 1.85 μmol/L), TBIL (15.08 ± 24.8 vs 13.16 ± 5.62μmol/L), WBC (7.58 ± 3.22 vs 5.66 ± 1.69 109/L), PLT (256.6 ± 102.96 vs 224.36 ± 56.79 109/L), these indicators were significantly higher in the high blood glucose group than in the normal blood glucose group. There were only 10 AEs (1.82%) among the 548 times undergoing WMT treatment, mainly diarrhea (3 patients, 0.55%), sore throat (2 patients, 0.36%), and anal pain (2 patients, 0.36%)), nausea (1 patient, 0.18%), dizziness (1 patient, 0.18%), joint soreness (1 patient, 0.18%). There were no serious AEs, and it resolved itself within a few days.
Table 2.
Demographics and clinical characteristics of patients at baseline.
| High blood glucose group (20) | Normal blood glucose group (175) | p-Value | |
|---|---|---|---|
| Age (year) | 54.8 ± 13.42 (n = 20) | 50.38 ± 15.29 (n = 175) | 0.275 |
| Male n (%) | 48.57 | 65 | 0.164 |
| BMI (kg/m2) | 24.75 ± 5.56 (n = 17) | 22.07 ± 3.94 (n = 174) | 0.423 |
| FBG (mmol/L) | 6.6 ± 1.67 (n = 20) | 4.62 ± 0.48 (n = 175) | <0.001 |
| HbA1c (%) | 6.02 ± 0.78 (n = 11) | 5.41 ± 0.30 (n=12) | 0.007 |
| FI (μU/mL) | 14.71 ± 10.86 (n = 11) | 8.12 ± 4.93 (n =94) | <0.001 |
| HOMA-IR | 4.26 ± 3.3 (n = 11) | 1.7 ± 1.1 (n = 94) | <0.001 |
| TC (mmol/L) | 5.24 ± 1.45 (n = 17) | 4.73 ± 1.03 (n = 157) | 0.206 |
| TG (mmol/L) | 3.33 ± 4.75 (n = 17) | 1.2 ± 0.78 (n = 157) | <0.001 |
| LDL-c (mmol/L) | 2.82 ± 0.94 (n = 17) | 2.87 ± 0.91 (n = 157) | 0.511 |
| HDL-c (mmol/L) | 1.09 ± 0.3 (n = 17) | 1.32 ± 0.32 (n = 157) | 0.827 |
| ApoB (g/L) | 1.04 ± 0.31 (n = 17) | 0.91 ± 0.23 (n = 157) | 0.741 |
| non-HDL-c (mmol/L) | 4.15 ± 1.65 (n = 17) | 3.4 ± 0.98 (n = 157) | 0.029 |
| LIP (mmol/L) | 193.34 ± 228.31 (n = 8) | 160.31 ± 235.92 (n = 49) | 0.366 |
| SBP (mmHg) | 130.2 ± 19.87 (n = 20) | 121.5 ± 13.08 (n = 175) | 0.130 |
| DBP (mmHg) | 78.75 ± 10.84 (n = 20) | 77.11 ± 9.7 (n = 175) | 0.592 |
| ALT (U/L) | 23.55 ± 13.14 (n = 20) | 20.54 ± 16.57 (n=174) | 0.817 |
| AST (U/L) | 26.1 ± 11.53 (n = 20) | 20.98 ± 7.12 (n = 174) | 0.015 |
| ALB (g/L) | 38.01 ± 6.29 (n = 20) | 40.7 ± 4.32 (n=174) | 0.307 |
| TP (g/L) | 67.87 ± 5.1 (n = 20) | 68.3 ± 6.01 (n = 174) | 0.219 |
| A/G | 1.33 ± 0.36 (n = 20) | 1.53 ± 0.33 (n = 174) | 0.972 |
| LAP (U/L) | 33.2 ± 8.93 (n = 12) | 29.71 ± 4.8 (n = 133) | 0.006 |
| GT (U/L) | 60.34 ± 80.04 (n = 11) | 23.88 ± 14.04 (n = 142) | <0.001 |
| GR (U/L) | 59.66 ± 16.49 (n = 17) | 56.51 ± 12.7 (n = 128) | 0.105 |
| DBIL (μmol/L) | 5.82 ± 10.41 (n = 20) | 4.25 ± 1.85 (n = 174) | <0.001 |
| IBIL (μmol/L) | 7.98 ± 7.87 (n = 20) | 8.83 ± 4.02 (n = 174) | 0.393 |
| TBIL (μmol/L) | 15.08 ± 24.8 (n = 20) | 13.16 ± 5.62 (n = 174) | 0.001 |
| RBC (1012/L) | 4.41 ± 0.8 (n = 20) | 4.32 ± 0.62 (n = 164) | 0.242 |
| HGB (g/L) | 130.05 ± 24.19 (n = 20) | 128.06 ± 18.22 (n = 164) | 0.222 |
| WBC (109/L) | 7.58 ± 3.22 (n = 20) | 5.66 ± 1.69 (n = 164) | 0.013 |
| LY (109/L) | 2 ± 0.71 (n = 20) | 1.74 ± 0.59 (n = 164) | 0.142 |
| PLT (109/L) | 256.6 ± 102.96 (n = 20) | 224.36 ± 56.79 (n = 164) | 0.021 |
Data presented as mean ± standard deviation, or n (%).
BMI (kg/m2), Body mass index; FBG (mmol/L), Fasting blood glucose; HbA1c (%), Glycated hemoglobin; FI (μU/mL), Fasting insulin; HOMA-IR, Homeostasis model assessment of insulin resistance; TC (mmol/L), Total cholesterol; TG (mmol/L), Triglyceride; LDL-c (mmol/L), Low-density lipoprotein cholesterol; HDL-c (mmol/L), High-density lipoprotein cholesterol; ApoB (g/L), Apolipoprotein B; non-HDL-c (mmol/L), Non-HDL cholesterol; LIP (mmol/L), Lipoprotein; SBP (mmHg), Systolic blood pressure; DBP (mmHg), Diastolic blood pressure; ALT (U/L), Alanine aminotransferase; AST (U/L), Aspartate aminotransferase; ALB (g/L), Serum albumin; TP (g/L), Total protein; A/G, Albumin/globulin; LAP (U/L), Leucine aminopeptidase; GT (U/L), glutamyl transpeptidase; GR (U/L), Glutathione Reductase; DBIL (μmol/L), Direct bilirubin; IBIL (μmol/L), Indirect bilirubin; TBIL (μmol/L), Total bilirubin; RBC (1012/L), Red blood cell; HGB (g/L), Hemoglobin; WBC (109/L), White blood cell; LY (109/L), Lymphocyte; PLT (109/L), Platelet.
Comparative analysis of each index after WMT treatment and baseline
Table 3 and Figure 2 show the effects of WMT on blood glucose, blood lipids, blood pressure, liver function and blood routine in patients with abnormal blood glucose metabolism. The results showed that WMT had a significant reducing effect on FBG in the short term (from 6.70 ± 1.65 to 5.29 ± 0.99mmol/L, p = 0.006) and medium term (from 6.58 ± 1.20 to 5.56 ± 0.95mmol/L, p = 0.025) in the high blood glucose group (p < 0.05), and also showed a decreasing effect in the long term (from 6.27 ± 0.08 to 4.80 ± 1.56mmol/L), but because the number of people was too small, it was not significant in the long term (p = 0.425). In the high blood glucose group, HbA1c decreased from 7.05 ± 0.49 to 6.95 ± 0.21mmol/L in the short term. FI decreased from 15.77 ± 10.3 to 11.88 ± 5.78 μU/mL in the short term and from 13.91 ± 11.8 to 11.98 ± 2.48 μU/mL in the long term. The HOMA-IR decreased from 4.73 ± 3.27 to 2.54 ± 1.36 in the short term and from 3.85 ± 3.25 to 2.64 ± 1.36 in the long term, but it was not significant. Overall, WMT has a good effect on improving blood glucose metabolism in patients with abnormal blood glucose metabolism.
Table 3.
The comparison values of each index in high blood glucose group and normal blood glucose group in the short term, medium term and long term with baseline during the treatment of washed microbiota transplantation.
| Items | Baseline | Short term | p-Value | Baseline | Medium term | p-Value | Baseline | Long term | p-Value |
|---|---|---|---|---|---|---|---|---|---|
| High blood glucose group | |||||||||
| BMI (kg/m2) | 24.75 ± 5.56 (n = 17) | 24.32 ± 5.37 (n = 17) | 0.303 | 23.54 ± 4.12 (n = 7) | 22.62 ± 4.26 (n = 7) | 0.067 | 21.11 ± 5.38 (n = 2) | 19.85 ± 3.25 (n = 2) | 0.557 |
| FBG (mmol/L) | 6.70 ± 1.65 (n = 18) | 5.29 ± 0.99 (n = 18) | 0.006 | 6.58 ± 1.20 (n = 8) | 5.56 ± 0.95 (n = 8) | 0.025 | 6.27 ± 0.08 (n = 2) | 4.80 ± 1.56 (n = 2) | 0.425 |
| HbA1c (%) | 7.05 ± 0.49 (n = 2) | 6.95 ± 0.21 (n = 2) | 0.874 | / | / | / | / | / | / |
| FI (μU/mL) | 15.77 ± 10.36 (n = 7) | 11.88 ± 5.78 (n = 7) | 0.359 | / | / | / | 13.91 ± 11.85 (n = 2) | 11.98 ± 2.48 (n = 2) | 0.820 |
| HOMA-IR | 4.73 ± 3.27 (n = 7) | 2.54 ± 1.36 (n = 7) | 0.134 | / | / | / | 3.85 ± 3.25 (n = 2) | 2.64 ± 1.36 (n = 2) | 0.532 |
| TC (mmol/L) | 5.36 ± 1.56 (n = 14) | 4.98 ± 1.09 (n = 14) | 0.338 | 6.68 ± 1.87 (n = 5) | 5.24 ± 1.09 (n = 5) | 0.136 | / | / | / |
| TG (mmol/L) | 3.78 ± 5.16 (n = 14) | 2.62 ± 3.03 (n = 14) | 0.198 | 7.22 ± 7.83 (n = 5) | 3.09 ± 3.21 (n = 5) | 0.043 | / | / | / |
| LDL-c (mmol/L) | 2.81 ± 1.00 (n = 14) | 2.86 ± 1.13 (n = 14) | 0.860 | 3.16 ± 1.29 (n = 5) | 2.78 ± 1.26 (n = 5) | 0.366 | / | / | / |
| HDL-c (mmol/L) | 1.06 ± 0.29 (n = 14) | 1.09 ± 0.27 (n = 14) | 0.575 | 0.83 ± 0.21 (n = 5) | 1.06 ± 0.21 (n = 5) | 0.082 | / | / | / |
| ApoB (g/L) | 1.07 ± 0.32 (n = 14) | 1.03 ± 0.20 (n = 14) | 0.676 | 1.28 ± 0.41 (n = 5) | 1.04 ± 0.15 (n = 5) | 0.314 | / | / | / |
| non-HDL-c (mmol/L) | 4.30 ± 1.75 (n = 14) | 3.90 ± 1.07 (n = 14) | 0.327 | 5.85 ± 2.05 (n = 5) | 4.18 ± 1.21 (n = 5) | 0.103 | / | / | / |
| LIP (mmol/L) | 198.64 ± 228.59 (n = 5) | 228.54 ± 250.33 (n = 5) | 0.168 | / | / | / | / | / | / |
| SBP (mmHg) | 130.20 ± 19.87 (n = 20) | 127.00 ± 20.83 (n = 20) | 0.315 | 124.11 ± 15.66 (n = 9) | 124.22 ± 14.47 (n = 9) | 0.984 | 128.67 ± 3.51 (n = 3) | 119.00 ± 7.00 (n = 3) | 0.048 |
| DBP (mmHg) | 78.75 ± 10.84 (n = 20) | 76.30 ± 11.47 (n = 20) | 0.275 | 75.22 ± 10.98 (n = 9) | 79.78 ± 9.50 (n = 9) | 0.243 | 75.33 ± 0.58 (n = 3) | 73.00 ± 5.20 (n = 3) | 0.556 |
| ALT (U/L) | 23.84 ± 13.43 (n = 19) | 24.21 ± 13.96 (n = 19) | 0.907 | 30.25 ± 13.66 (n = 8) | 22.75 ± 14.5 (n = 8) | 0.266 | 32.25 ± 13.15 (n = 4) | 23.00 ± 9.70 (n = 4) | 0.334 |
| AST (U/L) | 26.42 ± 11.75 (n = 19) | 26.84 ± 12.97 (n = 19) | 0.884 | 31.13 ± 13.44 (n = 8) | 24.00 ± 9.93 (n = 8) | 0.217 | 37.25 ± 17.23 (n = 4) | 36.75 ± 20.97 (n = 4) | 0.938 |
| ALB (g/L) | 38.00 ± 6.46 (n = 19) | 39.03 ± 5.54 (n = 19) | 0.455 | 36.08 ± 9.11 (n = 8) | 39.27 ± 8.03 (n = 8) | 0.220 | 32.33 ± 14.35 (n = 3) | 38.13 ± 13.11 (n = 3) | 0.058 |
| TP (g/L) | 67.94 ± 5.23 (n = 19) | 67.92 ± 5.58 (n = 19) | 0.991 | 65.41 ± 4.09 (n = 8) | 67.74 ± 6.93 (n = 8) | 0.385 | 62.17 ± 5.51 (n = 3) | 73.80 ± 8.25 (n = 3) | 0.121 |
| A/G | 1.33 ± 0.37 (n = 19) | 1.42 ± 0.39 (n = 19) | 0.323 | 1.34 ± 0.54 (n = 8) | 1.49 ± 0.53 (n = 8) | 0.435 | 1.26 ± 0.82 (n = 3) | 1.31 ± 0.85 (n = 3) | 0.586 |
| LAP (U/L) | 32.84 ± 9.27 (n = 11) | 33.16 ± 8.21 (n = 11) | 0.848 | 35.82 ± 11.72 (n = 5) | 36.68 ± 10.63 (n = 5) | 0.828 | 46.05 ± 13.93 (n = 2) | 49.35 ± 6.29 (n = 2) | 0.856 |
| GT (U/L) | 58.27 ± 84.05 (n = 10) | 47.62 ± 53.10 (n = 10) | 0.316 | 75.62 ± 119.66 (n = 5) | 54.08 ± 71.22 (n = 5) | 0.379 | 149.00 ± 197.99 (n = 2) | 102.70 ± 133.93 (n = 2) | 0.493 |
| GR (U/L) | 61.08 ± 15.92 (n = 16) | 59.23 ± 12.02 (n = 16) | 0.610 | 65.03 ± 18.02 (n = 6) | 56.18 ± 10.62 (n = 6) | 0.230 | 59.00 ± 31.11 (n = 2) | 65.50 ± 0.71 (n = 2) | 0.821 |
| DBIL (μmol/L) | 6.01 ± 10.66 (n = 19) | 5.87 ± 9.35 (n = 19) | 0.801 | 10.00 ± 16.08 (n = 8) | 6.96 ± 10.58 (n = 8) | 0.191 | 18.23 ± 27.08 (n = 3) | 12.72 ± 15.57 (n = 3) | 0.494 |
| IBIL (μmol/L) | 8.28 ± 7.97 (n = 19) | 8.22 ± 5.70 (n = 19) | 0.952 | 11.14 ± 11.98 (n = 8) | 11.43 ± 13.10 (n = 8) | 0.828 | 16.93 ± 20.45 (n = 3) | 14.75 ± 9.63 (n = 3) | 0.760 |
| TBIL (μmol/L) | 15.54 ± 25.39 (n = 19) | 14.09 ± 14.67 (n = 19) | 0.593 | 24.11 ± 38.83 (n = 8) | 18.39 ± 23.64 (n = 8) | 0.336 | 45.87 ± 64.33 (n = 3) | 19.83 ± 11.95 (n = 3) | 0.480 |
| RBC (1012/L) | 4.43 ± 0.82 (n = 19) | 4.38 ± 0.77 (n = 19) | 0.575 | 4.17 ± 1.03 (n = 9) | 4.52 ± 0.73 (n = 9) | 0.070 | 3.53 ± 1.30 (n = 4) | 4.40 ± 1.10 (n = 4) | 0.077 |
| HGB (g/L) | 132.42 ± 22.34 (n = 19) | 130.84 ± 16.43 (n = 19) | 0.577 | 123.44 ± 33.19 (n = 9) | 131.56 ± 23.08 (n = 9) | 0.203 | 103.00 ± 42.65 (n = 4) | 122.50 ± 32.28 (n = 4) | 0.246 |
| WBC (109/L) | 6.97 ± 1.76 (n = 19) | 6.04 ± 1.58 (n = 19) | 0.016 | 7.70 ± 4.33 (n = 9) | 5.77 ± 1.60 (n = 9) | 0.151 | 9.21 ± 6.63 (n = 4) | 5.7 ± 2.51 (n = 4) | 0.230 |
| LY (109/L) | 2.03 ± 0.71 (n = 19) | 1.93 ± 0.63 (n = 19) | 0.358 | 1.98 ± 0.62 (n = 9) | 1.63 ± 0.53 (n = 9) | 0.019 | 1.56 ± 0.33 (n = 4) | 2.03 ± 1.10 (n = 4) | 0.498 |
| PLT (109/L) | 238.47 ± 65.23 (n = 19) | 217.11 ± 58.10 (n = 19) | 0.037 | 275.89 ± 136.38 (n = 9) | 230.11 ± 106.85 (n = 9) | 0.031 | 346.00 ± 187.35 (n = 4) | 249.75 ± 118.42 (n = 4) | 0.099 |
| Normal blood glucose group | |||||||||
| BMI (kg/m2) | 22.09 ± 3.94 (n = 172) | 21.99 ± 3.89(n = 172) | 0.386 | 21.7 ± 3.67(n = 96) | 21.51 ± 3.7 (n = 96) | 0.375 | 22.07 ± 3.99 (n = 45) | 21.98 ± 4.04(n = 45) | 0.787 |
| FBG (mmol/L) | 4.63 ± 0.48 (n = 154) | 4.59 ± 0.78 (n = 154) | 0.538 | 4.63 ± 0.5 (n = 86) | 4.54 ± 0.82(n = 86) | 0.261 | 4.77 ± 0.55 (n = 42) | 4.79 ± 0.83 (n = 42) | 0.893 |
| HbA1c (%) | 5.15 ± 0.50 (n = 2) | 5.20 ± 0.71 (n = 2) | 0.795 | / | / | / | / | / | / |
| FI (μU/mL) | 8.52 ± 5.31 (n=53) | 8.80 ± 5.46 (n=53) | 0.624 | 8.98 ± 5.48 (n=29) | 8.95 ± 5.8 (n=29) | 0.960 | 11.49 ± 6.36 (n=15) | 10.97 ± 6.7 (n=15) | 0.667 |
| HOMA-IR | 1.77 ± 1.16 (n=53) | 1.87 ± 1.45 (n=53) | 0.489 | 1.90 ± 1.28 (n=29) | 1.95 ± 1.44 (n=29) | 0.729 | 2.46 ± 1.5 (n=15) | 2.33 ± 1.57 (n=15) | 0.615 |
| TC (mmol/L) | 4.76 ± 1.06 (n=110) | 4.66 ± 1.03 (n=110) | 0.206 | 4.72 ± 1.06 (n=63) | 4.58 ± 0.93 (n=63) | 0.197 | 4.94 ± 1.12 (n=29) | 4.61 ± 1 (n=29) | 0.039 |
| TG (mmol/L) | 1.31 ± 0.84 (n=110) | 1.19 ± 0.69 (n=110) | 0.030 | 1.33 ± 0.9 (n=63) | 1.5 ± 2.03 (n=63) | 0.449 | 1.42 ± 0.79 (n=29) | 1.29 ± 0.96 (n=29) | 0.259 |
| LDL-c (mmol/L) | 2.88 ± 0.93 (n=110) | 2.82 ± 0.89 (n=110) | 0.402 | 2.79 ± 0.9 (n=63) | 2.64 ± 0.82 (n=63) | 0.124 | 2.92 ± 0.99 (n=29) | 2.69 ± 0.88 (n=29) | 0.099 |
| HDL-c (mmol/L) | 1.3 ± 0.33 (n=110) | 1.31 ± 0.31 (n=110) | 0.631 | 1.35 ± 0.34 (n=63) | 1.32 ± 0.3 (n=63) | 0.456 | 1.38 ± 0.31 (n=29) | 1.34 ± 0.31 (n=29) | 0.150 |
| ApoB (g/L) | 0.92 ± 0.24 (n=110) | 0.91 ± 0.24 (n=110) | 0.412 | 0.91 ± 0.23 (n=63) | 0.90 ± 0.25 (n=63) | 0.705 | 0.96 ± 0.21 (n=29) | 0.96 ± 0.24 (n=29) | 0.796 |
| non-HDL-c (mmol/L) | 3.46 ± 1.01 (n=110) | 3.35 ± 0.98 (n=110) | 0.148 | 3.38 ± 0.97 (n=63) | 3.25 ± 0.87 (n=63) | 0.225 | 3.57 ± 0.99 (n=29) | 3.28 ± 0.92 (n=29) | 0.049 |
| LIP (mmol/L) | 156.14 ± 303.68 (n=23) | 156.29 ± 278.95 (n=23) | 0.986 | 77.49 ± 75.35 (n=10) | 83.04 ± 78.93 (n=10) | 0.444 | 60.35 ± 24.68 (n=2) | 42.60 ± 2.12 (n=2) | 0.521 |
| SBP (mmHg) | 121.26 ± 12.75 (n=174) | 120.38 ± 11.79 (n=174) | 0.389 | 120.16 ± 12.85 (n=98) | 119.6 ± 11.94 (n=98) | 0.708 | 120.87 ± 12.11 (n=45) | 119.49 ± 10.09 (n=45) | 0.499 |
| DBP (mmHg) | 76.91 ± 9.38 (n=174) | 76.44 ± 9.12 (n=174) | 0.566 | 76.38 ± 9.47 (n=98) | 74.86 ± 8.31 (n=98) | 0.168 | 76.80 ± 9.41 (n=45) | 77.16 ± 8.03 (n=45) | 0.823 |
| ALT (U/L) | 20.65 ± 16.8 (n=168) | 19.55 ± 13.2 (n=168) | 0.355 | 20.25 ± 15.25 (n=88) | 20.5 ± 13.32 (n=88) | 0.863 | 19.91 ± 11.46 (n=43) | 21.12 ± 18.55 (n=43) | 0.633 |
| AST (U/L) | 21.04 ± 7.22 (n=168) | 21.29 ± 7.59 (n=168) | 0.671 | 20.78 ± 6.35 (n=88) | 21.80 ± 7.36 (n=88) | 0.201 | 21.26 ± 6.09 (n=43) | 23.33 ± 12.34 (n=43) | 0.177 |
| ALB (g/L) | 40.71 ± 4.36 (n=168) | 40.53 ± 3.88 (n=168) | 0.562 | 40.93 ± 4.77 (n=88) | 40.36 ± 4.06 (n=88) | 0.205 | 40.52 ± 5.01 (n=43) | 40.4 ± 4.18 (n=43) | 0.880 |
| TP (g/L) | 68.34 ± 5.99 (n=168) | 67.79 ± 5.75 (n=168) | 0.234 | 68.41 ± 6.44 (n=88) | 67.63 ± 5.67 (n=88) | 0.231 | 68.65 ± 5.97 (n=43) | 67.64 ± 5.48 (n=43) | 0.226 |
| A/G | 1.53 ± 0.33 (n=168) | 1.53 ± 0.28 (n=168) | 0.995 | 1.56 ± 0.38 (n=88) | 1.52 ± 0.3 (n=88) | 0.368 | 1.51 ± 0.41 (n=43) | 1.52 ± 0.32 (n=43) | 0.869 |
| LAP (U/L) | 29.71 ± 5 (n=112) | 30.25 ± 5.01 (n=112) | 0.047 | 29.36 ± 4.86 (n=63) | 29.69 ± 5.57 (n=63) | 0.340 | 29.41 ± 5.45 (n=31) | 29.62 ± 5.14 (n=31) | 0.672 |
| GT (U/L) | 24.17 ± 14.4 (n=121) | 24.33 ± 14.97 (n=121) | 0.789 | 24.98 ± 15.38 (n=70) | 24.77 ± 16.11 (n=70) | 0.838 | 27.85 ± 14.00 (n=35) | 26.35 ± 12.68 (n=35) | 0.318 |
| GR (U/L) | 56.34 ± 12.92 (n=119) | 56.96 ± 13.46 (n=119) | 0.340 | 54.24 ± 10.62 (n=62) | 54.65 ± 11.74 (n=62) | 0.682 | 53.3 ± 11.8 (n=28) | 53.47 ± 13.1 (n=28) | 0.912 |
| DBIL (μmol/L) | 4.19 ± 1.79 (n = 168) | 4.16 ± 2.04 (n = 168) | 0.761 | 4.13 ± 1.71 (n = 88) | 4.08 ± 1.87 (n = 88) | 0.769 | 4.09 ± 1.71 (n = 43) | 4.15 ± 1.57 (n = 43) | 0.826 |
| IBIL (μmol/L) | 8.72 ± 3.77 (n = 168) | 8.47 ± 3.97 (n = 168) | 0.336 | 8.75 ± 3.79 (n = 88) | 8.42 ± 3.95 (n = 88) | 0.326 | 9.19 ± 4.07 (n = 43) | 8.96 ± 3.91 (n = 43) | 0.694 |
| TBIL (μmol/L) | 13.01 ± 5.3 (n = 168) | 12.64 ± 5.8 (n = 168) | 0.287 | 12.94 ± 5.26 (n = 88) | 12.51 ± 5.62 (n = 88) | 0.357 | 13.29 ± 5.62 (n = 43) | 13.10 ± 5.32 (n = 43) | 0.819 |
| RBC (1012/L) | 4.32 ± 0.62 (n = 164) | 4.29 ± 0.62 (n = 164) | 0.143 | 4.32 ± 0.54 (n = 85) | 4.31 ± 0.56 (n = 85) | 0.721 | 4.31 ± 0.56 (n = 39) | 4.34 ± 0.55 (n = 39) | 0.628 |
| HGB (g/L) | 128.06 ± 18.22 (n = 164) | 127.06 ± 18.19 (n = 164) | 0.140 | 127.21 ± 19.15 (n = 85) | 127.46 ± 16.85 (n = 85) | 0.843 | 127.23 ± 21.56 (n = 39) | 128.49 ± 18.46 (n = 39) | 0.476 |
| WBC (109/L) | 5.66 ± 1.69 (n = 164) | 5.84 ± 2.12 (n = 164) | 0.178 | 5.60 ± 1.64 (n = 85) | 5.31 ± 1.58 (n = 85) | 0.021 | 5.62 ± 1.64 (n = 39) | 5.34 ± 1.47 (n = 39) | 0.209 |
| LY (109/L) | 1.74 ± 0.59 (n = 164) | 1.77 ± 0.66 (n = 164) | 0.392 | 1.69 ± 0.58 (n = 85) | 1.74 ± 0.57 (n=85) | 0.337 | 1.66 ± 0.54 (n = 39) | 1.68 ± 0.64 (n = 39) | 0.794 |
| PLT (109/L) | 224.36 ± 56.79 (n=164) | 222.84 ± 58.66 (n=164) | 0.536 | 220.73 ± 57.45 (n=85) | 212.55 ± 46.86 (n=85) | 0.042 | 223.56 ± 64.26 (n = 39) | 211.77 ± 52.94 (n = 39) | 0.111 |
Data presented as mean ± standard deviation, or n (%).
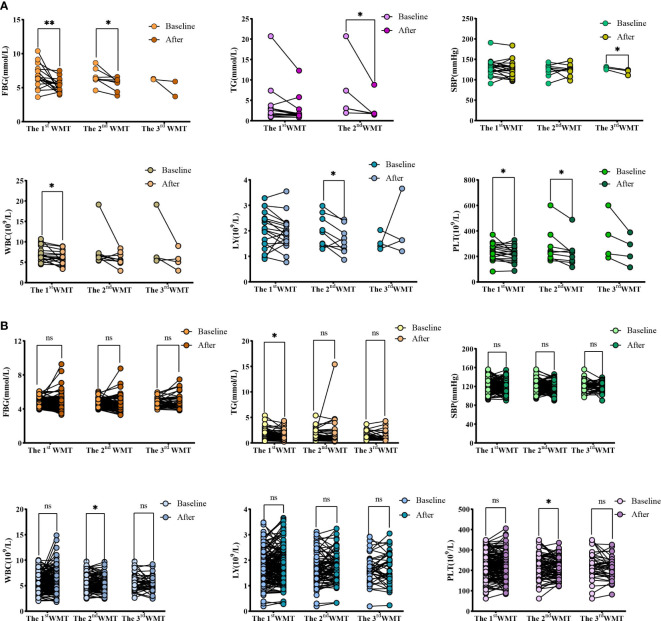
Figure 2.
Changes of FBG, TG, SBP, WBC, LY and PLT levels after 1-3 times of washing and transplanting. (A) Changes of FBG, TG, SBP, WBC, LY and PLT in high blood glucose group; (B) Changes of FBG, TG, SBP, WBC, LY and PLT in normal blood glucose group. FBG, Fasting blood glucose; TG, Triglyceride; SBP, Systolic blood pressure; WBC, White blood cell; LY, Lymphocyte; PLT, Platelet. * indicates p < 0.05 ** indicates p < 0.01; ns, not significant.
At the same time, in the high blood glucose group, WMT significantly reduced TG (from 7.22 ± 7.83 to 3.09 ± 3.21mmol/L, p = 0.043) in the mid-term (p < 0.05), indicating that WMT has the effect of reducing blood lipids. There was a significant long term reduction (p < 0.05) on SBP (from 128.67 ± 3.51 to 119.00 ± 7.00mmHg, p = 0.048), indicating that WMT has a blood pressure lowering effect. There was a significant short term reduction (p < 0.05) on WBC (from 6.97 ± 1.76 to 6.04 ± 1.58 109/L, p = 0.016), indicating that WMT may have the function of inhibiting inflammatory response. Significantly decreased LY (from 1.98 ± 0.62 to 1.63 ± 0.53 109/L, p = 0.019) in the medium term (p < 0.05), indicating that WMT may have an immunomodulatory effect. There was a significant reduction (p < 0.05) in the short term (from 238.47 ± 65.23 to 217.11 ± 58.10 109/L, p = 0.037) and mid-term (from 275.89 ± 136.38 to 230.11 ± 106.85 109/L, p = 0.031) of PLT, indicating that WMT may have a role in regulating cardiovascular disease. There was no significant change in FBG, HbA1c, FI, HOMA-IR, etc. in the short term, medium term and long term in the normal blood glucose group by WMT, that is, WMT had no effect on normoglycemic patients. But for TC (p = 0.039) and TG (p = 0.030) were significantly decreased (p < 0.05). Both the high blood glucose group and the normal blood glucose group had a decreasing trend in BMI before and after treatment, but there was no significant change.
Correlation analysis of WMT on indicators affecting blood glucose regulation
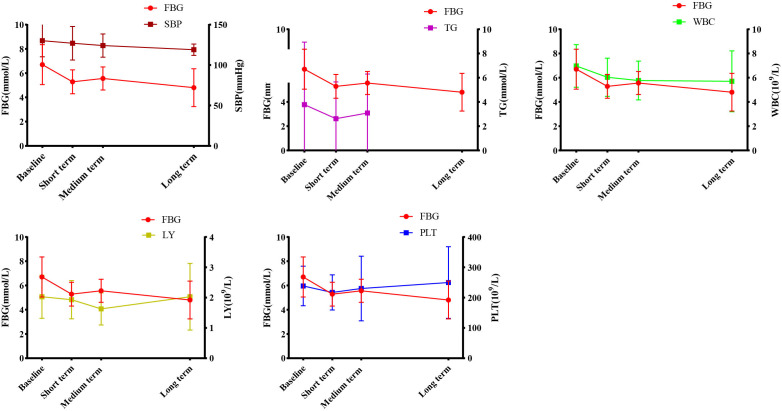
Earlier we found that during the treatment process, WMT had a significant improvement effect on FBG, TG, SBP, WBC, LY and PLT in the high blood glucose group. In order to find the relevant factors that affect the regulation of blood glucose by WMT, correlation analysis was carried out on the above-mentioned indicators with significant regulation effect. As shown in Figure 3 , we found that in high blood glucose levels, FBG was positively correlated with TG (r=0.973), SBP (r=0.866), WBC (r=0.926), and LY (r=0.089); FBG was negatively correlated with PLT (r=-0.069). Our data show that during WMT treatment, blood pressure, blood lipids, anti-inflammatory response and immune function are simultaneously affected while improving blood glucose, and there is an interaction link. This provides us with a good treatment idea for the treatment of patients with abnormal blood glucose metabolism, that is, in addition to lowering blood glucose, it can also assist in the treatment of blood pressure and blood lipids, inhibit inflammation and improve immunity while treating high blood glucose.
Figure 3.
Correlation analysis of WMT on blood glucose regulation. FBG, Fasting blood glucose; TG, Triglyceride; SBP, Systolic blood pressure; WBC, White blood cell; LY, Lymphocyte; PLT, Platelet.
Evaluation of WMT clinical therapeutic effect on blood glucose levels
All the enrolled patients were divided into high blood glucose group and normal blood glucose group according to blood glucose baseline. Patients were regrouped according to changes in blood glucose levels after 1-3 WMT treatments ( Table 4 ). There were significant changes in blood glucose levels in the short term in patients with high blood glucose group. In the high blood glucose group, 72.22% returned to normal in the short term (p < 0.001), 57.14% returned to normal in the medium term (p = 0.076), and long term returned to normal is not significant (p = 0.317). Because the number of people is too small, there is no significant difference in the long term. Our data suggest that WMT can significantly change higher blood glucose levels in patients to normal levels in short term treatment; however, the efficacy of WMT remains to be explored. Our data show that WMT may significantly improve blood glucose levels after short term treatment, whereas blood glucose levels stabilize after medium and long term treatment.
Table 4.
Comprehensive clinical efficacy of short, medium and long term treatment on blood glucose levels.
| Data periods | Before therapy (n) | Therapeutic effect base on blood glucose levels | |||
|---|---|---|---|---|---|
| Unchanged group (n) | Changed group (n, %) | X2 | p-Value | ||
| High blood glucose group | |||||
| Short term | 18 | 5 | 13 (72.22%) | 17.338 | <0.001 |
| Medium term | 7 | 3 | 4 (57.14%) | 3.150 | 0.076 |
| Long term | 2 | 0 | 2 (100%) | 1.000 | 0.317 |
| Normal blood glucose group | |||||
| Short term | 154 | 149 | 5(3.36%) | 3.253 | 0.071 |
| Medium term | 86 | 83 | 3 (4.65%) | 1.375 | 0.244 |
| Long term | 42 | 37 | 5 (13.51%) | 3.403 | 0.065 |
The definition of unchanged and changed of high blood glucose group were still high blood glucose group and changed to normal.
The definition of unchanged and changed of normal blood glucose were still normal and changed to high blood glucose group.
Evaluation of the therapeutic effect of WMT on the ASCVD risk
According to ASCVD risk stratification, patients were divided into extremely high risk group, high risk group, medium risk group and low risk group. After WMT treatment, patients were regrouped into no risk changed group and the risk-changed group ( Table 5 ). Acute coronary syndrome, coronary heart disease, stroke, and peripheral atherosclerosis were included in the extremely high risk group, and these patients were not reassigned after WMT and were not listed in Table 5 .
Table 5.
Effects of short, medium, and long term treatment on atherosclerotic cardiovascular disease risk classification.
| Data periods | Before therapy (n) | Therapeutic effect base on ASCVD risk stratification | |||
|---|---|---|---|---|---|
| Unchangedgroup (n) | Changedgroup (n, %) | X2 | p-Value | ||
| High risk group | |||||
| Short term | 24 | 18 | 6(25%) | 4.792 | 0.029 |
| Medium term | 15 | 10 | 5(33.3%) | 3.896 | 0.050 |
| Long term | 7 | 6 | 1(14.3%) | 1.077 | 0.299 |
| Medium risk group | |||||
| Short term | 10 | 8 | 2(20%) | 0.556 | 0.456 |
| Medium term | 6 | 5 | 1(16.7%) | 1.091 | 0.296 |
| Long term | 5 | 4 | 1(20%) | 1.111 | 0.292 |
| Low risk group | |||||
| Short term | 84 | 79 | 5(5.8%) | 3.298 | 0.069 |
| Medium term | 44 | 43 | 1(2.3%) | 1.011 | 0.315 |
| Long term | 19 | 17 | 2(10.5%) | 0.528 | 0.468 |
The definition of unchanged of high-, medium-, and low-risk groups were still high-, medium-, and low-risk after WMT procedures, respectively.
In the high-risk group, the effects of short term and medium term WMT treatment were significant. Short term 25% and medium term 33.3% were classified as medium risk group or below (p < 0.05). In the medium risk group, the effects of short, medium and long term WMT treatment were not significant, but the number of people was reduced. In the ASCVD low risk group, this change was not statistically significant, suggesting that WMT does not increase the risk of ASCVD.
Discussion
To the best of our knowledge, this is one of the few clinical study to investigate the effects of WMT on patients with abnormal blood glucose metabolism, especially those with high blood glucose, in South China, indicating that regulating gut microbiota may be a new method for the treatment of abnormal blood glucose metabolism. These data show that WMT has a significant short and medium term effect on improving blood glucose in patients with high blood glucose, and at the same time, it has significant hypolipidemic and blood pressure-lowering effects on blood lipids and blood pressure in patients with high blood glucose. Overall, WMT significantly reduced the risk of high risk ASCVD in the short and medium term, and there was no significant difference between the medium and low risk groups, suggesting that WMT could reduce the short and medium term risk of ASCVD without increasing the risk of ASCVD.
Numerous studies have shown that gut microbiota disturbances may influence the progression of diabetes (32, 33). Patients with T2DM have a moderate dysbiosis between butyrate-producing and lactic acid bacteria (34). Furthermore, various metabolites, such as short chain fatty acids (SCFAs), produced by the gut microbiota differ significantly between T2DM and normal hosts (35). Studies have shown that ingestion of probiotics can effectively improve gut microbiota disturbances and relieve symptoms in diabetic patients, such as remodeling the formation of gut microbiota to improve or control the disease state (36). FMT is an approach to treat disease by rebuilding the microbiota (37). There is growing evidence that the therapeutic potential of FMT is based on an established clinical program that has become the first-line treatment for recurrent Clostridium difficile infections (38). However, understanding the impact of gut microbiota on metabolic disease is still in its infancy, and data on the effect of FMT on T2DM are still scarce. In our study, WMT had a significant short and medium term improvement in blood glucose in patients with high blood glucose. It is possible to restore gut microbiota balance to promote host homeostasis (39).
The potential effects of FMT on glucose homeostasis and insulin sensitivity in humans have been identified. FMT using lean donors was sufficient to improve glucose homeostasis in obese individuals, with a small decrease in HbA1c 6 weeks after FMT, which was associated with changes in gut microbiota (40). Another FMT double-blind placebo-controlled trial reported a slight improvement in HbA1c at 12 weeks (41). Our results for FBG and HbA1c showed a decreasing trend, providing further evidence for the hypoglycemic effect of WMT. As reported in the literature, FMT can show a restored phenotype following transfer of the donor’s gut microbiota to the recipient (42). T2DM mice had reduced FI levels and improved HOMA-IR after reconstitution of their microbiota from normal mouse feces (17). Likewise, in a clinical trial, obese patients treated with FMT in lean healthy individuals showed positive changes in homeostasis model assessment of insulin sensitivity (HOMA-IS) (43). Our study did not observe a significant improvement in FI and HOMA-IR after WMT, although some studies showed that FMT significantly improved HOMA-IS (44). Our non-significant results may be due to the small sample size in our study, the lack of HOMA-IR indicators in many of our high blood glucose patients, and due to technical factors including the accuracy of the instrument, among others. For the current study, gut dysbiosis was associated with the development of insulin resistance and diabetes (42). The mechanism of gut microbiota-induced improvement in insulin resistance may be through altering body energy balance or reducing obesity induced by a high-fat diet. However, the exact mechanism needs to be fully explored.
FMT is associated with low-grade inflammation characterized by metabolic disturbances. Therapeutic FMT has been reported to reduce the secretion of inflammatory factors and trigger multiple immune-mediated signaling pathways in colitis (45). As mentioned in one study, transplantation of gut microbiota such as Faecalibacterium prausnitzii prevented inflammatory damage to the pancreas (46). These findings are consistent with our results, the WBC in the high blood glucose group after WMT showed a decreasing trend in the short, medium and long term, and there were significant differences in the short term, indicating that WMT may inhibit the inflammatory response. LY showed a significant downward trend in the medium term, indicating that WMT may have an immunomodulatory effect. IL-6 and TNF-α are pro-inflammatory cytokines with multiple functions that can directly act on islet cells and cause islet β-cell damage (47). Low-grade chronic inflammation caused by microbiota imbalance usually leads to islet structural damage and impaired islet β-cell function, and islet β-cell apoptosis is the underlying cause of islet structural damage. Therefore, FMT treatment of inflammatory response and apoptosis of islet β cells can reverse islet damage and dysfunction, which may be a method worth exploring.
T2DM patients often have one or more components of metabolic syndrome, such as hypertension, dyslipidemia, obesity, etc., which significantly increase the risk, progression speed and harm of T2DM complications. Comprehensive treatment should be based on the control of blood glucose, blood pressure, blood lipids and body weight, antiplatelet therapy and other lifestyle improvements. In our study, in addition to improving blood glucose, WMT has blood lipid-lowering and blood pressure-lowering effects in patients with high blood glucose, and has a significant reduction in platelets in the short and medium term, which is in line with the recommendations of antiplatelet therapy. We speculate that the improvement of abnormal blood glucose metabolism after WMT is due to the improvement of gut microbiota after WMT, which comprehensively regulates blood lipids, blood pressure, and platelets. The short term goal of diabetes treatment is to eliminate diabetes symptoms and prevent acute complications by controlling hyperglycemia and metabolic disorders. The long term goal of diabetes treatment is to prevent chronic complications, improve patients’ quality of life and prolong life through good metabolic control. A scientific and reasonable T2DM treatment strategy should be comprehensive, including control of blood glucose, blood pressure, blood lipids, and body weight, antiplatelet therapy, and lifestyle improvements. Although there are several studies on WMT function, such as Liang et al. showed that WMT treatment can alter blood lipids in patients with hyperlipidemia and hypolipidemia without serious adverse events (48). Studies by Zhong et al. have shown that WMT has antihypertensive effects in hypertensive patients (49). Pan et al. showed that WMT significantly improved children with autism spectrum disorder, gastrointestinal symptoms and sleep disturbance, and reduced systemic inflammation (50). The mechanism of FMT-induced disease remission remains largely unexplained. Diabetic symptoms may be alleviated by synergistic effects between gut commensal microbiota after FMT treatment, or may trigger multiple immune-inflammatory processes and pathways.
Future research should focus on the bacterial species and functional changes associated with FMT treatment in diabetic patients and how FMT affects the metabolism of other organs in long term improvement. Due to the complexity of the gut microbiota, further studies should explore whether specific microbial species or communities in FMT are dedicated to the prevention and treatment of diabetes. This may provide a new perspective and reference. Many factors influence the outcome of FMT, namely donor selection and preparation, sample handling, mode of administration, and colonization resistance (51). FMT-related AEs are a challenge for FMT applications. In most cases, mild gastrointestinal AEs were well tolerated by FMT (52). Our WMT project based on Zhang’s criteria found no serious AEs during and after WMT. Zhang et al. prepared washed microbial flora by repeating centrifugation and suspension three times on the basis of an automatic purification system, which significantly reduced AEs (25). At present, the understanding of the effect of WMT on the gut microbiota on metabolic diseases is still in its infancy, and data on the effect of WMT on abnormal blood glucose metabolism are still lacking. This is the first large-scale retrospective trial of abnormal blood glucose metabolism in China, including high blood glucose group and normal blood glucose group. The clinical evidence of the effect of WMT on blood glucose metabolism has been established, which lays the foundation for subsequent studies on the effects of gut microbiota (53), metabolic markers (54), and diet (55) regulation on abnormal blood glucose metabolism. Taken together, these results suggest that restoring gut microbiota may serve as a promising treatment for abnormal blood glucose metabolism; however, the mechanism of action requires further investigation.
This study has several limitations. First, this study mainly focused on the analysis of clinical blood glucose metabolism, blood lipid metabolism, and liver function, etc. The gut microbiota metagenomics and metabolomics before and after WMT have not been evaluated. Therefore, the lowering effect of WMT on blood glucose and related microbiota is unclear. Second, the impact of patient compliance. A small number of patients did not undergo long term treatment after short and medium term treatment, and returned to the hospital at short or long intervals for evaluation of the long term benefit of WMT treatment. Therefore, more data are needed to confirm the long term efficacy of WMT in the treatment of abnormal blood glucose metabolism. Third, we did not consider potential confounding factors between the main symptoms of WMT treatment and glycemia. Although data show that WMT can improve abnormal blood glucose metabolism in the short and medium term, we need large-scale prospective studies to further validate our conclusions.In the future, we plan to conduct a large-sample prospective study to verify the effect of WMT on blood glucose metabolism.
Conclusion
WMT has a significant effect of improving blood glucose, and at the same time, it has a significant effect on reducing blood lipid and blood pressure in patients with high blood glucose. WMT reduces the short- and medium-term risk of ASCVD without increasing the long term risk of ASCVD. Therefore, the regulation of gut microbiota by WMT may provide a new clinical approach for the treatment of abnormal blood glucose metabolism.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee (No. 2017-98) in accordance with the Declaration of Helsinki at the First Affiliated Hospital of Guangdong Pharmaceutical University, Guangzhou, China. The patients/participants provided their written informed consent to participate in this study.
Author contributions
X-XH, Q-PW, and LW designed the concept of the study. M-QL, Y-TX, X-JL, QZ, W-YL, and J-TX collected and analyzed the data. TL and W-YL were the statistics consultant, QZ was the consultant for endocrinology. LW wrote the draft manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Key Technologies R&D Program of Guangdong Province (2022B1111070006), the Natural Science Foundation of Guangdong Province (2019A1515010125), and the Department of Education of Guangdong Province (2021KCXTD025).
Acknowledgments
We sincerely thank all patients in the study and all funding agencies that supported the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci Rep (2020) 10(1):14790. doi: 10.1038/s41598-020-71908-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dwyer-Lindgren L, Mackenbach JP, van Lenthe FJ, Flaxman AD, Mokdad AH. Diagnosed and undiagnosed diabetes prevalence by county in the U.S., 1999–2012. Diabetes Care (2016) 39(9):1556–62. doi: 10.2337/dc16-0678 [DOI] [PubMed] [Google Scholar]
- 3. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF diabetes atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract (2018) 138:271–81. doi: 10.1016/j.diabres.2018.02.023 [DOI] [PubMed] [Google Scholar]
- 4. Diabetes Society of Chinese Medical Association . Guideline for the prevention and treatment of type 2 diabetes mellitus in China (2020 edition) (Part 1). Chin J of Pract Intern Med (2021) 41(8):668–95. doi: 10.19538/j.nk2021080106 [DOI] [Google Scholar]
- 5. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA (2017) 317(24):2515–23. doi: 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia W, Weng J, Zhu D, Ji L, Lu J, Zhou Z, et al. Society: Standards of medical care for type 2 diabetes in China 2019. Diabetes/Metabolism Res Rev (2019) 35(6):e3158. doi: 10.1002/dmrr.3158 [DOI] [PubMed] [Google Scholar]
- 7. The L. Diabetes: a dynamic disease. Lancet (2017) 389(10085):2163. doi: 10.1016/S0140-6736(17)31537-4 [DOI] [PubMed] [Google Scholar]
- 8. Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, et al. Mortality attributable to diabetes in 20–79 years old adults, 2019 estimates: Results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Res Clin Pract (2020) 162:108086. doi: 10.1016/j.diabres.2020.108086 [DOI] [PubMed] [Google Scholar]
- 9. Krentz AJ, Patel MB, Bailey CJ. New drugs for type 2 diabetes mellitus. Drugs (2008) 68(15):2131–62. doi: 10.2165/00003495-200868150-00005 [DOI] [PubMed] [Google Scholar]
- 10. Cani PD. Human gut microbiome: hopes, threats and promises. Gut (2018) 67(9):1716. doi: 10.1136/gutjnl-2018-316723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: An integrative view. Cell (2012) 148(6):1258–70. doi: 10.1016/j.cell.2012.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sedighi M, Razavi S, Navab-Moghadam F, Khamseh ME, Alaei-Shahmiri F, Mehrtash A, et al. Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. (2017) 111:362–9. doi: 10.1016/j.micpath.2017.08.038 [DOI] [PubMed] [Google Scholar]
- 13. Pedersen HK, Gudmundsdottir V, Nielsen HB, Hyotylainen T, Nielsen T, Jensen BAH, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature (2016) 535(7612):376–81. doi: 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- 14. Zhao L, Zhang F, Ding X, Wu G, Lam Yan Y, Wang X, et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science (2018) 359(6380):1151–6. doi: 10.1126/science.aao5774 [DOI] [PubMed] [Google Scholar]
- 15. Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J (2013) 7(4):880–4. doi: 10.1038/ismej.2012.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang C, Yin A, Li H, Wang R, Wu G, Shen J, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine (2015) 2(8):968–84. doi: 10.1016/j.ebiom.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang H, Lu Y, Yan Y, Tian S, Zheng D, Leng D, et al. Promising treatment for type 2 diabetes: Fecal microbiota transplantation reverses insulin resistance and impaired islets. Front Cell Infect Microbiol (2020) 9:455. doi: 10.3389/fcimb.2019.00455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bafeta A, Yavchitz A, Riveros C, Batista R, Ravaud P. Methods and reporting studies assessing fecal microbiota transplantation. Ann Internal Med (2017) 167(1):34–9. doi: 10.7326/M16-2810 [DOI] [PubMed] [Google Scholar]
- 19. de Groot P, Scheithauer T, Bakker GJ, Prodan A, Levin E, Khan MT, et al. Donor metabolic characteristics drive effects of faecal microbiota transplantation on recipient insulin sensitivity, energy expenditure and intestinal transit time. Gut (2020) 69(3):502. doi: 10.1136/gutjnl-2019-318320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qazi T, Amaratunga T, Barnes EL, Fischer M, Kassam Z, Allegretti JR. The risk of inflammatory bowel disease flares after fecal microbiota transplantation: Systematic review and meta-analysis. Gut Microbes (2017) 8(6):574–88. doi: 10.1080/19490976.2017.1353848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allegretti JR, Kassam Z, Mullish BH, Chiang A, Carrellas M, Hurtado J, et al. Effects of fecal microbiota transplantation with oral capsules in obese patients. Clin Gastroenterol Hepatol (2020) 18(4):855–63. doi: 10.1016/j.cgh.2019.07.006 [DOI] [PubMed] [Google Scholar]
- 22. Kootte RS, Levin E, Salojärvi J, Smits LP, Hartstra AV, Udayappan SD, et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab (2017) 26(4):611–619.e6. doi: 10.1016/j.cmet.2017.09.008 [DOI] [PubMed] [Google Scholar]
- 23. Cruz-Aguliar RM, Wantia N, Clavel T, Vehreschild MJGT, Buch T, Bajbouj M, et al. An open-labeled study on fecal microbiota transfer in irritable bowel syndrome patients reveals improvement in abdominal pain associated with the relative abundance of akkermansia muciniphila. Digestion (2019) 100(2):127–38. doi: 10.1159/000494252 [DOI] [PubMed] [Google Scholar]
- 24. Pamer EG. Fecal microbiota transplantation: Effectiveness, complexities, and lingering concerns. Mucosal Immunol (2014) 7(2):210–4. doi: 10.1038/mi.2013.117 [DOI] [PubMed] [Google Scholar]
- 25. Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, et al. Washed microbiota transplantation vs. manual fecal microbiota transplantation: Clinical findings, animal studies and in vitro screening. Protein Cell (2020) 11(4):251–66. doi: 10.1007/s13238-019-00684-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang F. Fecal microbiota transplantation-standardization study: Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J (2020) 133(19):2330–2. doi: 10.1097/CM9.0000000000000954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mullish BH, Quraishi MN, Segal JP, McCune VL, Baxter M, Marsden GL, et al. The use of fecal microbiota transplant as treatment for recurrent or refractory clostridium difficile infection and other potential indications: Joint British society of gastroenterology (BSG) and healthcare infection society (HIS) guidelines. Gut (2018) 67(11):1920. doi: 10.1136/gutjnl-2018-316818 [DOI] [PubMed] [Google Scholar]
- 28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 29. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. Practice guidelines for the management of arterial hypertension of the European society of cardiology and the European society of hypertension: ESC/ESH task force for the management of arterial hypertension. J Hypertens. (2018) 36(12):314–40. doi: 10.1080/08037051.2018.1527177 [DOI] [PubMed] [Google Scholar]
- 30. Chinese Cardiovascular Disease Prevention Guidelines (2017) Writing Group, Editorial Board of Chinese Journal of Cardiovascular Diseases . Chinese Guidelines for the prevention of cardiovascular diseases. Chin J Cardiol (2018) 46(1):10–25. doi: 10.3760/cma.j.issn.0253-3758.2018.01.004 [DOI] [PubMed] [Google Scholar]
- 31. Chinese Medical Association General Practice Branch . Guideline for primary care of obesity: Practice version (2019). Chin J Gen Pract (2020) 19(2):102–7. doi: 10.3760/cma.j.issn.1671-7368.2020.02.003 [DOI] [Google Scholar]
- 32. Nie Q, Chen H, Hu J, Fan S, Nie S. Dietary compounds and traditional Chinese medicine ameliorate type 2 diabetes by modulating gut microbiota. Crit Rev Food Sci Nutr (2019) 59(6):848–63. doi: 10.1080/10408398.2018.1536646 [DOI] [PubMed] [Google Scholar]
- 33. Saad MJA, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology (2016) 31(4):283–93. doi: 10.1152/physiol.00041.2015 [DOI] [PubMed] [Google Scholar]
- 34. Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature (2012) 490(7418):55–60. doi: 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 35. Salek RM, Maguire ML, Bentley E, Rubtsov DV, Hough T, Cheeseman M, et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol Genomics (2007) 29(2):99–108. doi: 10.1152/physiolgenomics.00194.2006 [DOI] [PubMed] [Google Scholar]
- 36. Shen T-CD, Albenberg L, Bittinger K, Chehoud C, Chen Y-Y, Judge CA, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest (2015) 125(7):2841–50. doi: 10.1172/JCI79214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee P, Yacyshyn BR, Yacyshyn MB. Gut microbiota and obesity: An opportunity to alter obesity through faecal microbiota transplant (FMT). Diabetes Obes Metab (2019) 21(3):479–90. doi: 10.1111/dom.13561 [DOI] [PubMed] [Google Scholar]
- 38. Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, et al. European Consensus conference on faecal microbiota transplantation in clinical practice. Gut (2017) 66(4):569. doi: 10.1136/gutjnl-2016-313017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Meslier V, Laiola M, Roager HM, De Filippis F, Roume H, Quinquis B, et al. Mediterranean Diet intervention in overweight and obese subjects lowers plasma cholesterol and causes changes in the gut microbiome and metabolome independently of energy intake. Gut (2020) 69(7):1258. doi: 10.1136/gutjnl-2019-320438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rinott E, Youngster I, Yaskolka Meir A, Tsaban G, Zelicha H, Kaplan A, et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology (2021) 160(1):158–173.e10. doi: 10.1053/j.gastro.2020.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu EW, Gao L, Stastka P, Cheney MC, Mahabamunuge J, Soto MT, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PloS Med (2020) 17(3):e1003051. doi: 10.1371/journal.pmed.1003051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M. Fecal microbiota transplantation in metabolic syndrome: History, present and future. Gut Microbes (2017) 8(3):253–67. doi: 10.1080/19490976.2017.1293224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aron-Wisnewsky J, Clément K, Nieuwdorp M. Fecal microbiota transplantation: A future therapeutic option for Obesity/Diabetes? Curr Diabetes Rep (2019) 19(8):51. doi: 10.1007/s11892-019-1180-z [DOI] [PubMed] [Google Scholar]
- 44. Vrieze A, Van Nood E, Holleman F, Salojärvi J, Kootte RS, Bartelsman JFWM, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology (2012) 143(4):913–916.e7. doi: 10.1053/j.gastro.2012.06.031 [DOI] [PubMed] [Google Scholar]
- 45. Burrello C, Garavaglia F, Cribiù FM, Ercoli G, Lopez G, Troisi J, et al. Therapeutic faecal microbiota transplantation controls intestinal inflammation through IL10 secretion by immune cells. Nat Commun (2018) 9(1):5184. doi: 10.1038/s41467-018-07359-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ganesan K, Chung SK, Vanamala J, Xu B. Causal relationship between diet-induced gut microbiota changes and diabetes: A novel strategy to transplant faecalibacterium prausnitzii in preventing diabetes. Int J Mol Sci (2018) 19(12):3720. doi: 10.3390/ijms19123720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park J, Decker JT, Smith DR, Cummings BJ, Anderson AJ, Shea LD. Reducing inflammation through delivery of lentivirus encoding for anti-inflammatory cytokines attenuates neuropathic pain after spinal cord injury. J Controlled Release (2018) 290:88–101. doi: 10.1016/j.jconrel.2018.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang F, Lu X, Deng Z, Zhong HJ, Zhang W, Li Q, et al. Effect of washed microbiota transplantation on patients with dyslipidemia in south China. Front Endocrinol (2022) 13:827107. doi: 10.3389/fendo.2022.827107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhong HJ, Zeng HL, Cai YL, Zhuang Y-P, Liou YL, Wu Q, et al. Washed microbiota transplantation lowers blood pressure in patients with hypertension. Front Cell Infect Microbiol (2021) 11:679624. doi: 10.3389/fcimb.2021.679624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pan ZY, Zhong HJ, Huang DN, Wu LH, He XX. Beneficial effects of repeated washed microbiota transplantation in children with autism. Front Pediatr (2022) 10:928785. doi: 10.3389/fped.2022.928785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Leshem A, Horesh N, Elinav E. Fecal microbial transplantation and its potential application in cardiometabolic syndrome. Front Immunol (2019) 10:1341. doi: 10.3389/fimmu.2019.01341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang SA, Xu MQ, Wang WQ, Cao XC, Piao MY, Khan S, et al. Systematic review: Adverse events of fecal microbiota transplantation. PloS One (2016) 11(8):e0161174. doi: 10.1371/journal.pone.0161174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu L, Xie X, Li Y, Liang T, Zhong H, Ma J, et al. Metagenomics-based analysis of the age-related cumulative effect of antibiotic resistance genes in gut microbiota. Antibiotics (2021) 10(8):1006. doi: 10.3390/antibiotics10081006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu L, Xie X, Liang T, Ma J, Yang L, Yang J, et al. Integrated multi-omics for novel aging biomarkers and antiaging targets. Biomolecules (2022) 12(1):39. doi: 10.3390/biom12010039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liang T, Wu L, Xi Y, Li Y, Xie X, Fan C, et al. Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit Rev Food Sci Nutr (2021) 61(10):1670–88. doi: 10.1080/10408398.2020.1764488 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.