FIGURE 3.
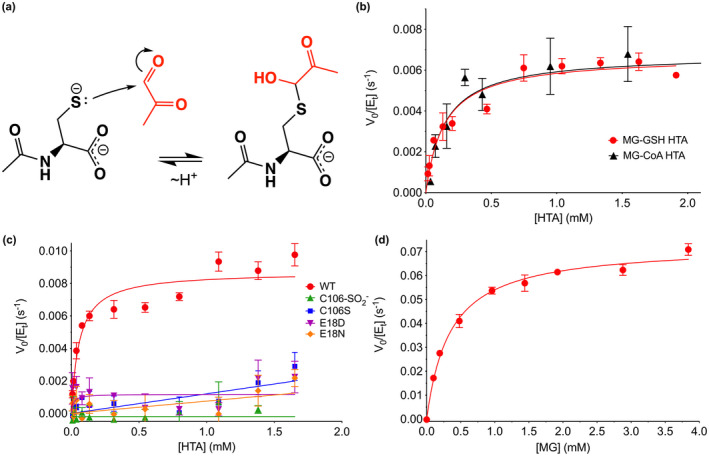
DJ‐1 has apparent hemithioacetal deglycase and glyoxalase activities in vitro. (a) Mechanism of formation of hemithioacetal formation by NAC (black) and MG (red). The hemithioacetal is reversible, as indicated by the arrows. (b) Steady‐state enzyme kinetics of DJ‐1 acting on the MG‐glutathione (MG‐GSH; red) and MG‐coenzyme a (MG‐CoA; black) hemitioacetal substrates. (c) Steady‐state kinetics of DJ‐1 acting on the MG‐N‐acetyl cysteine (MG‐NAC) hemithioacetal (HTA) substrate. Wild‐type (WT) enzyme is in red, and mutants and oxidative modification are shown in the inset legend with the indicated symbols and colors. Only WT DJ‐1 is active enough to be reliably fitted using the Michaelis–Menten model (solid lines). (d) Steady‐state kinetics of DJ‐1 glyoxalase activity against MG as substrate. In panels (b–d), initial velocity (V 0) is divided by total enzyme concentration ([Et]) on the Y‐axis, rates were measured a minimum of three times with standard deviation shown in bars, and the fitted Michaelis–Menten curves are shown as solid lines.
