Abstract
Seasonal influenza is a frequent cause of hospitalization and mortality among patients with systemic autoimmune diseases. Despite this evidence, vaccination coverage is generally much lower than the minimum 75% target proposed by the WHO. Therefore, an active campaign was implemented in the years 2019/2020 and 2020/2021 within the Rheumatology Department of the Niguarda Hospital (Milan, Italy) to improve the vaccination coverage in patients with inflammatory arthritis. This study aims to evaluate the vaccination coverage in the 2019/2020 and 2020/2021 (active campaigns) seasons and to compare these results with the 2018/2019 season. A monocenter observational study was conducted among adult patients with rheumatoid arthritis, spondylarthritis, or psoriatic arthropathy, who were referred to the Rheumatology Department of the Niguarda Hospital. Patients were given a questionnaire to investigate previous years’ vaccination coverage and to propose an influenza vaccine for the 2020/2021 season. Compared with 2018/2019, a trend for increase in vaccination coverage was reported in 2019/2020 season (+ 10.7%, p = 0.055; 45.5% of coverage) and a statistically significant increase was reported in 2020/2021 (+ 31.2%, p < 0.001; 65.9% of coverage). The increase was also significant when comparing the 2020/2021 and 2019/2020 seasons (+ 20.5%, p < 0.001). The greatest increase in vaccination coverage was observed among under-65-year-old patients. Obtained results support the implementation of active vaccination campaigns to increase vaccination coverage among patients with systemic autoimmune diseases and highlight the importance of external factors (such as the COVID-19 pandemic) in directing the patient to adopt preventive measures to avoid infections and related complications.
|
Key Points • Vaccination coverage is generally much lower than the minimum 75% target proposed by WHO among patients with systemic autoimmune diseases. • To improve the vaccination coverage, an active campaign was implemented in the years 2019/2020 and 2020/2021 within the Rheumatology Department of the Niguarda Hospital (Milan, Italy). • Compared with 2018/2019, a trend for increase in vaccination coverage was reported in 2019/2020 season (+ 10.7%, p = 0.055; 45.5% of coverage) and a significant increase was reported in 2020/2021 (+ 31.2%, p < 0.001; 65.9% of coverage) season. • Obtained results support the implementation of active vaccination campaigns to increase vaccination coverage among patients with systemic autoimmune diseases. |
Keywords: Active vaccination campaign; Influenza; Psoriatic arthropathy, Rheumatoid arthritis; Spondylarthritis; Systemic autoimmune diseases; Vaccination
Introduction
Patients with systemic autoimmune diseases are at increased risk of influenza and severe respiratory complications, particularly if they are on therapy with immunosuppressive drugs belonging to the biological and synthetic last-generation drug classes [1–3]. Respiratory tract infections are the most common in this patient setting; among them, seasonal influenza remains one of the most frequent causes of hospitalization and mortality, similar to that observed in the general population [1, 4–6].
Vaccines represent one of the safest and most effective means of disease control [7, 8]. For this reason, influenza vaccination is strongly recommended in most patients with inflammatory arthritis according to the 2011 and 2019 European Alliance of Associations for Rheumatology (EULAR) guidelines and 2019 Italian guidelines [3, 9, 10].
Different influenza vaccine formulations have been shown to ensure the development of proper titers of protective antibodies against influenza in numerous cohorts of patients with inflammatory arthritis, despite ongoing immunosuppressive therapy [6, 11, 12]. The influenza vaccine was found safe in this patient setting, and the benefits outweighed the hypothetical risks associated with the vaccination itself [13, 14].
Despite this evidence of efficacy and safety, seasonal influenza vaccination coverage in patients with inflammatory arthritis is generally much lower than the minimum 75% target proposed by the World Health Organization (WHO) [15–17].
It has been proposed that a direct link with the vaccine providers or the introduction of influenza vaccination as a routine practice in rheumatology outpatient wards may be a useful approach to improve vaccination coverage [6, 18]. In line with this observation and following the scientific reference societies’ recommendations, an active vaccination campaign was implemented in the years 2019/2020 and 2020/2021 within the clinical practice of the Rheumatology Department of the Niguarda Hospital (Milan, Italy) to increase the seasonal influenza vaccination coverage in patients with inflammatory arthritis.
On a practical level, the active vaccination campaign was implemented by offering patients the possibility of carrying out influenza vaccination before the routine drug withdrawal or infusion of biological drugs, rather than limiting the vaccination recommendation as done in previous years (e.g., 2018/2019). After the second year of the active vaccination campaign, the purpose of this observational monocentric study has been to assess the variation of the vaccination coverage among patients on the latest generation of biological and synthetic immunosuppressive drugs for systemic autoimmune diseases in the 2019/2020 and 2020/2021 vaccination seasons (active vaccination campaigns) and to compare these results with the 2018/2019 vaccination season.
Patients and methods
Study design and setting
This monocenter observational study involved > 18-year-old patients with rheumatoid arthritis, spondylarthritis, or psoriatic arthropathy, who were referred to the Rheumatology Department of Niguarda Hospital (Milan, Italy) for drug withdrawal or infusion of biological drugs in the period between 12/11/2020 and 18/1/2021. The only exclusion criterion was the patient’s refusal to participate in the study.
Patients were given a questionnaire to investigate the vaccination coverage in previous years and to propose influenza vaccination for the current season (further details about the questionnaire in the following paragraph). In addition to the questionnaire answers, the following information was collected through the evaluation of medical records: patient socio-demographic data, diagnosis, and ongoing therapy.
This study was conducted according to the ethical standards established by the 1964 Declaration of Helsinki and with the protocol approved by the Milano Area 3 ethics committee (register number 289–20,042,022). All the participants signed an informed consent form.
Survey structure
The questionnaire included the following six questions:
Did you carry out the influenza vaccination in the 2018/2019 winter season?
Did you carry out the influenza vaccination in the 2019/2020 winter season?
If you answered “Yes” to question 2, where did you carry out the vaccination? (general practitioner, Niguarda Hospital, another center).
Did you carry out the influenza vaccination in the current season (2020/2021)?
Did you plan to carry out the influenza vaccination this year (2020/2021)?
Would you like to carry out the influenza vaccination immediately after the rheumatological examination at our department?
Study assessments
The study’s primary objective was to quantify vaccination coverage in the 2018/2019, 2019/2020, and 2020/2021 seasons.
The change in vaccination coverage in the 2019/2020 season compared with the 2018/2019 season and the change in vaccination coverage in the 2020/2021 season compared with the 2019/2020 and 2018/2019 seasons were considered secondary outcomes. An exploratory analysis was conducted to evaluate the vaccination status and the relative variations between vaccination seasons among patients stratified by age (cut-off was 65 years).
Statistical analysis
The point estimate and the interval estimate were calculated (95% CI with the exact method and Wald approximation) by considering patients reporting to have been vaccinated in the single vaccination season (numerator) and the total number of patients assessed in terms of vaccination status (denominator). This was done separately for each vaccination season and in the following comparisons between seasons: 2020/2021 vs 2018/2019, 2020/2021 vs 2019/2020, and 2019/2020 vs 2018/2019. The same estimates were evaluated among patients stratified by age (above or below 65 years). The Stata 2017 software was used for the analysis. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.
Results
Among 553 eligible patients, 391 (70.7%) were enrolled and filled in the questionnaire. The mean (standard deviation) age was 57 (13) years, and 129 patients (33.0%) were ≥ 65 years. Table 1 shows the characteristics of enrolled patients.
Table 1.
Characteristics of enrolled patients (n = 391)
| Characteristics | n (%) |
|---|---|
| Females | 259 (66.2) |
| Diagnosis: | |
| • Rheumatoid arthritis | 221 (56.5) |
| • Psoriatic arthritis | 64 (16.4) |
| • Spondylarthritis | 67 (17.1) |
| • Other diagnoses | 39 (10.0) |
| Therapy: | |
| • Glucocorticoids: | |
| o ≥ 7.5 mg/die prednisone equivalent | 38 (9.7) |
| • csDMARD: | |
| o Methotrexate | 89 (22.8) |
| o Hydroxychloroquine | 27 (6.9) |
| o Other | 23 (5.9) |
| • bDMARD: | |
| o Etanercept | 83 (21.2) |
| o Adalimumab | 86 (22.0) |
| o Golimumab | 32 (8.2) |
| o Certolizumab | 17 (4.3) |
| o Infliximab | 10 (2.6) |
| o Tocilizumab | 44 (11.2) |
| o Sarilumab | 11 (2.8) |
| o Abatacept | 48 (12.3) |
| o Anakinra | 3 (0.8) |
| o Rituximab | 7 (1.8) |
| o Secukinumab | 19 (4.9) |
| • tsDMARDs: | |
| o Tofacitinib | 11 (2.8) |
| o Baricitinib | 6 (1.5) |
| o Upadacitinib | 1 (0.3) |
| o Apremilast | 4 (1.0) |
csDMARD conventional synthetic disease–modifying antirheumatic drugs, bDMARD biological disease-modifying antirheumatic drugs, tsDMARD targeted synthetic disease-modifying antirheumatic drugs
Seasonal influenza vaccination coverage
In 2018/2019 vaccination season, 34.7% (95% CI: 26.6–43.2; n = 136) of patients were vaccinated. This percentage increased to 45.5% (95% CI: 38.0–53.1; n = 178) in 2019/2020 and further increased to 65.9% (95% CI: 59.8–71.6; n = 258) in the 2020/2021 season.
In the 2019/2020 season, 101 (25.8%) patients were vaccinated by their general practitioner, 44 (11.6%) at the Niguarda Hospital, and 33 (8.4%) at another center.
In the 2020/2021 season, 149 (38.1%) patients had already been vaccinated by their general practitioner, and 89 (22.8%) reported they had already scheduled vaccination and intended to carry it out at the Niguarda Hospital. Following the proposal to carry out the vaccine at the Niguarda Hospital, 23 (5.9%) patients joined the vaccination campaign. Three patients then renounced vaccination between completing the questionnaire and performing it. Overall, 109 vaccinations were performed at the Niguarda Hospital in the 2020/2021 season (27.8%).
Secondary outcomes
Seasonal influenza vaccination coverage variations
In the 2020/2021 vaccination season, there was a significant increase in vaccination coverage compared with both 2018/2019 (+ 31.2%; p < 0.001) and 2019/2020 (+ 20.5; p < 0.001) seasons (Table 2).
Table 2.
Vaccination coverage variations
| Vaccination season | Vaccinated patients | Variations from the previous seasons, % (95% CI) | p-value | |
|---|---|---|---|---|
| n | % (95% CI) | |||
| 2018/2019 | 136 | 34.7 (26.6–43.2) | – | – |
| 2019/2020 | 178 | 45.5 (38.0–53.1) | + 10.7 (− 0.1–21.6) | p = 0.055 |
| 2020/2021 | 258 | 65.9 (59.8–71.6) |
+ 20.5 (11.1–29.8)* + 31.2 (21.3–41.1)** |
p < 0.001 p < 0.001 |
*vs 2019/20; ** vs 2018/19
The increase in vaccination coverage in the 2019/2020 season compared with 2018/2019 was not statistically significant (+ 10.7%; p = 0.055; Table 2).
Variations in seasonal influenza vaccination coverage according to the age of patients
Vaccination coverage was highest among ≥ 65-year-old patients (n = 129, 33%) over the three considered seasons (Table 3). However, although vaccination coverage was significantly higher in over-65-year-old patients in the 2018/2019 and 2019/2020 seasons, this statistically significant difference was lost in the 2020/2021 season (Table 3).
Table 3.
Influenza vaccination coverage according to the age of patients
| Season | Under-65-year-old vaccinated patients, n = 262 (67.0) | Over-65-year-old vaccinated patients, n = 129 (33.0) | p-value | ||
|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | ||
| 2018/2019 | 65 | 24.8 (14.77–36,87) | 71 | 55.0 (42.67–66.78) | < 0.001 |
| 2019/2020 | 99 | 37.8 (27.85–47.67) | 79 | 61.2 (49.12–71.56) | 0.002 |
| 2020/2021 | 165 | 62.9 (55.18–70.40) | 93 | 72.1 (61.78–80.86) | 0.137 |
The increase in vaccination coverage mainly involved the under-65-year-old population, with a significant increase in the 2020/2021 season compared with both 2018/2019 (+ 38.2%, 95% CI: 25.34–51.00; p < 0.001) and 2019/2020 (+ 25.2%, 95% CI: 13.13–37.25; p < 0.001; Fig. 1). However, the increase in vaccination coverage in the over-65-year-old group was only significant when comparing the 2020/2021 and 2018/2019 seasons (+ 17.0%, 95% CI: 2.32–31.78; p < 0.001; Fig. 1).
Fig. 1.
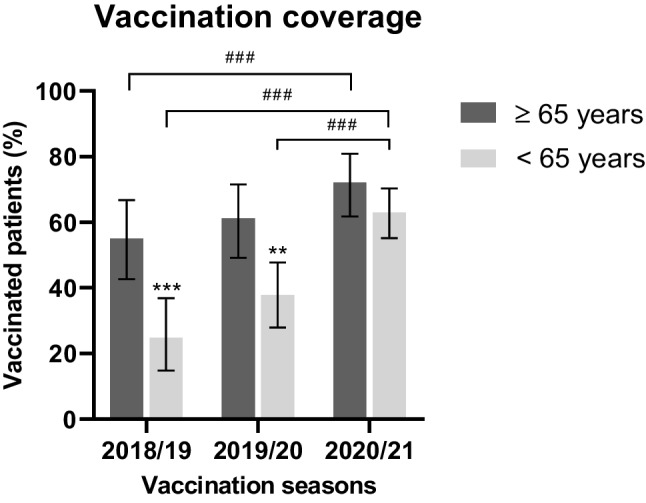
Percentage of vaccinated patients in the seasons 2018/2019, 2019/2020, and 2020/2021 according to the age of patients. Vaccination coverage was significantly higher in over-65-year-old patients (n = 129, 33%) in the 2018/2019 and 2019/2020 seasons. Vaccination coverage among under-65-year-old patients (n = 262, 67%) increased significantly in 2020/2021 compared with both 2019/2020 and 2018/2019. Vaccination coverage among ≥ 65-year-old patients increased significantly in 2020/2021 compared with 2018/2019. *Inter-group comparisons; #Intra-group comparisons. Statistical significance: **p < 0.01; ***.,###p < 0.001
Discussion
Improving vaccination rates among patients suffering from systemic autoimmune diseases is a public health priority as it may enhance protection in this patient setting and the whole community [19]. Several factors were associated with the low vaccination rates among this group of patients; most were related to high perceived vaccine risk and the low perceived efficacy [20, 21]. In addition, provider-related and healthcare system–related factors were also identified, such as vaccine hesitancy (a catch-all category of delay and/or refusal of vaccine uptake and potential decision-making category) and promotion of opinions and actions against vaccination use [22, 23].
The results of this study suggest that the possibility of carrying out the seasonal influenza vaccination at the rheumatology referral center is a useful tool to increase vaccination coverage in patients with inflammatory arthritis on drug therapy with hospital distribution. Indeed, compared with 2018/2019, a trend for increase in vaccination coverage was reported in 2019/2020 (+ 10.7%, p = 0.055; 45.5% of vaccination coverage) season and a statistically significant increase was reported in the 2020/2021 (+ 31.2%, p < 0.001; 65.9% of vaccination coverage) vaccination season. Although not meeting WHO’s proposed objectives (75% of vaccination coverage), in this experience, the achieved higher uptake of influenza vaccination may be related to a positive and proactive attitude toward vaccination from rheumatologists.
Notably, the increase in vaccination coverage was also significant when comparing the 2020/2021 and 2019/2020 seasons (+ 20.5%, p < 0.001), both active vaccination seasons. This suggests that external factors may have further sensitized patients to the importance of vaccinations. As previously observed, the most important factor has been the COVID-19 pandemic, which has certainly increased patients’ awareness of the possible consequences of an airway infection during immunosuppressive therapy and, more generally, has stimulated the search for vaccine information [21, 24]. Accordingly, during the COVID-19 pandemic, a strong desire for vaccination was reported among patients with rheumatic diseases. In contrast, concerns about adverse events or exacerbations of rheumatic disease were observed in a lower proportion [25]. Also, the impact of isolation measures applied to patients with febrile symptoms and airway infection must be considered. Indeed, during the COVID-19 pandemic, patients with febrile symptoms were required to remain isolated for certain periods and to perform frequent swabs to confirm the absence of SARS-CoV-2 infection. It can be assumed that influenza vaccination, able to reduce the chance of febrile symptoms, has taken on a new value for reducing the risk of facing restrictive measures for COVID-19.
The greatest increase in vaccination coverage was observed among under-65-year-old patients. In addition to the reasons listed above closely related to the COVID-19 pandemic, the active vaccination campaign may have played a decisive role in this subgroup of patients. Indeed, only over-65-year-old patients are generally actively recruited by general practitioners in Italy to carry out the influenza vaccination. For under-65-year-old patients, the vaccination is free, but it must be the patient to request it. Since the active vaccination campaign involved both under- and over-65-year-old patients in our center, this may have contributed to reducing the difference in vaccination coverage between the two patient groups.
Previous data assessing the global results of different interventions to improve vaccine acceptance among rheumatoid arthritis patients reported an overall increase in vaccination coverage of + 8.4 ± 13.6%. For instance, this study assessed only information and reminder activities without evaluating studies providing the possibility of performing influenza vaccination before the routine visit [19]. Our results related to the 2019/2020 season (+ 10.7%) are in line with these data; otherwise, the additional increase observed in the 2020/2021 season (+ 20.5% over 2019/2020) further supports our observation about the possible role of external factors.
Conclusion
Despite the limitations of this study due to the retrospective and monocentric nature, obtained results suggest that the rheumatologist’s active role can improve vaccination coverage among patients with inflammatory arthritis, particularly those under the age of 65 years. This study also suggests the importance of external factors (such as the COVID-19 pandemic) in directing the patient with systemic autoimmune diseases on the possibility of adopting measures to prevent possible infections and related complications. Overall, the results of this experience support the implementation of active vaccination campaigns to increase vaccination coverage.
Acknowledgements
Editorial assistance was provided by Simonetta Papa, PhD; Valentina Attanasio; and Aashni Shah (Polistudium SRL, Milan, Italy).
Author contribution
Study design: Michel Chevallard, Nicola Ughi, Oscar Massimiliano Epis; data collection and interpretation: all; manuscript writing: Michel Chevallard, Nicola Ughi; manuscript editing: all; approval to submit: all.
Data availability
Data may be made available upon reasonable request.
Compliance with ethical standards
Ethics approval
The study was conducted within the protocol approved by the Milano Area 3 ethics committee (register number 289–20042022).
Consent to participate
All the participants signed an informed consent form.
Consent for publication
Not required as this manuscript does not include details, images, or videos related to the participants.
Conflict of interests
None.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Milanovic MS, Kadijevich DM, Stojanovich L, Milovanovic B, Djokovic A. A lower level of post-vaccinal antibody titer against influenza virus A H1N1 may protect patients with autoimmune rheumatic diseases from respiratory viral infections. Medicina (Kaunas) 2022;58:76. doi: 10.3390/medicina58010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trombetta CM, Gianchecchi E, Montomoli E. Influenza vaccines: evaluation of the safety profile. Hum Vaccin Immunother. 2018;14:657–670. doi: 10.1080/21645515.2017.1423153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, Breedveld FC, D'Amelio R, Dougados M, Kapetanovic MC, van Laar JM, de Thurah A, Landewé RB, Molto A, Müller-Ladner U, Schreiber K, Smolar L, Walker J, Warnatz K, Wulffraat NM, Elkayam O. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79:39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 4.Bower H, Frisell T, Di Giuseppe D, Delcoigne B, Askling J. Influenza outcomes in patients with inflammatory joint diseases and DMARDs: how do they compare to those of COVID-19? Ann Rheum Dis. 2022;81(3):433–439. doi: 10.1136/annrheumdis-2021-221461. [DOI] [PubMed] [Google Scholar]
- 5.Papiris SA, Manali ED, Kolilekas L, Kagouridis K, Maniati M, Filippatos G, Bouros D. Acute respiratory events in connective tissue disorders. Respiration. 2016;91:181–201. doi: 10.1159/000444535. [DOI] [PubMed] [Google Scholar]
- 6.Murdaca G, Noberasco G, Olobardi D, Lunardi C, Maule M, Delfino L, Triggiani M, Cardamone C, Benfaremo D, Moroncini G, Vacca A, Susca N, Gangemi S, Quattrocchi P, Sticchi L, Icardi G, Orsi A. Current take on systemic sclerosis patients’ vaccination recommendations. Vaccines (Basel) 2021;9:1426. doi: 10.3390/vaccines9121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plotkin S, Orenstein W, Offit P (2008) Vaccines. Lancet Infect Dis 8 (Elsevier)
- 8.Pollard AJ, Bijker EM. A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol. 2021;21:83–100. doi: 10.1038/s41577-020-00479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Assen S, Agmon-Levin N, Elkayam O, Cervera R, Doran MF, Dougados M, Emery P, Geborek P, Ioannidis JP, Jayne DR, Kallenberg CG, Müller-Ladner U, Shoenfeld Y, Stojanovich L, Valesini G, Wulffraat NM, Bijl M. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–422. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 10.Guerrini G, Franzetti F, Giacomelli R, Meroni L, Riva A, Scirè CA, Scrivo R, Tavio M, Agostinone A, Airò P, Atzeni F, Bartalesi F, Bazzichi L, Berardicurti O, Cassola G, Castagna A, Castelli F, Cattelan A, Citriniti G, Cristini F, De Rosa F, Fracassi E, Galloway J, La Paglia GMC, Moioli MC, Ripamonti D, Saracino A, Tani C, Tascini C, Tieghi T, Tinelli M, Zabotti A, Sarzi-Puttini P, Galli M. Italian recommendations for influenza and pneumococcal vaccination in adult patients with autoimmune rheumatic diseases. Clin Exp Rheumatol. 2020;38:245–256. doi: 10.55563/clinexprheumatol/hj69ne. [DOI] [PubMed] [Google Scholar]
- 11.Meroni PL, Zavaglia D, Girmenia C. Vaccinations in adults with rheumatoid arthritis in an era of new disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol. 2018;36:317–328. [PubMed] [Google Scholar]
- 12.Stapleton JT, Wagner N, Tuetken R, Bellamy AR, Hill H, Kim S, Winokur PL. High dose trivalent influenza vaccine compared to standard dose vaccine in patients with rheumatoid arthritis receiving TNF-alpha inhibitor therapy and healthy controls: Results of the DMID 10–0076 randomized clinical trial. Vaccine. 2020;38:3934–3941. doi: 10.1016/j.vaccine.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agmon-Levin N, Kivity S, Shoenfeld Y. Influenza vaccine and autoimmunity. Isr Med Assoc J. 2009;11:183–185. [PubMed] [Google Scholar]
- 14.Fernández R-F, de Larrinoa Í, Carreira PE, Brito García N, Del Campo D, Fontecha P, Pego Reigosa JM, Gómez Puerta JA, Ortega-Castro R, Tejera Segura B, Aguado García JM, Torre-Cisneros J, Valencia-Martín JL, Pereda CA, Nishishinya-Aquino MB, Otón Sánchez MT, Silva Fernández L, Maese Manzano J, Chamizo Carmona E, Correyero Plaza M. Recommendations for prevention of infection in systemic autoimmune rheumatic diseases. Reumatol Clin (Engl Ed) 2021 doi: 10.1016/j.reuma.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Assala M, Groh M, Blanche P, Vinter C, Cohen P, Le Guern V, Puéchal X, Mouthon L, Le Jeunne C, Launay O, Kernéis S. Pneumococcal and influenza vaccination rates in patients treated with corticosteroids and/or immunosuppressive therapies for systemic autoimmune diseases: a cross-sectional study. Joint Bone Spine. 2017;84(3):365–366. doi: 10.1016/j.jbspin.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 16.Hmamouchi I, Winthrop K, Launay O, Dougados M. Low rate of influenza and pneumococcal vaccine coverage in rheumatoid arthritis: data from the international COMORA cohort. Vaccine. 2015;33:1446–1452. doi: 10.1016/j.vaccine.2015.01.065. [DOI] [PubMed] [Google Scholar]
- 17.Subesinghe S, Rutherford AI, Ibrahim F, Harris H, Galloway J. A large two-centre study in to rates of influenza and pneumococcal vaccination and infection burden in rheumatoid arthritis in the UK. BMC Musculoskelet Disord. 2016;17:322. doi: 10.1186/s12891-016-1187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosamilia F, Noberasco G, Olobardi D, Orsi A, Icardi G, Lantieri F, Murdaca G. Flu and pneumococcal vaccine coverage in scleroderma patients still need to be prompted: a systematic review. Vaccines (Basel) 2021;9:1330. doi: 10.3390/vaccines9111330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosselin Boucher V, Colmegna I, Gemme C, Labbe S, Pelaez S, Lavoie KL. Interventions to improve vaccine acceptance among rheumatoid arthritis patients: a systematic review. Clin Rheumatol. 2019;38:1537–1544. doi: 10.1007/s10067-019-04430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams WW, Lu PJ, O’Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, Rodriguez-Lainz A, Fiebelkorn AP. Surveillance of vaccination coverage among adult populations — United States, 2015. MMWR Surveill Summ. 2017;66:1–28. doi: 10.15585/mmwr.ss6611a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fragoulis GE, Grigoropoulos I, Mavrea E, Arida A, Bournia VK, Evangelatos G, Fragiadaki K, Karamanakos A, Kravvariti E, Panopoulos S, Pappa M, Thomas K, Tektonidou MG, Paraskevis D, Vassilopoulos D, Sfikakis PP. Increased influenza vaccination rates in patients with autoimmune rheumatic diseases during the Covid-19 pandemic: a cross-sectional study. Rheumatol Int. 2021;41:895–902. doi: 10.1007/s00296-021-04817-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boucher VG, Pelaez S, Gemme C, Labbe S, Lavoie KL. Understanding factors associated with vaccine uptake and vaccine hesitancy in patients with rheumatoid arthritis: a scoping literature review. Clin Rheumatol. 2021;40:477–489. doi: 10.1007/s10067-020-05059-7. [DOI] [PubMed] [Google Scholar]
- 23.Peretti-Watel P, Larson HJ, Ward JK, Schulz WS, Verger P (2015) Vaccine hesitancy: clarifying a theoretical framework for an ambiguous notion. PLoS Curr 7:ecurrents.outbreaks.6844c80ff9f5b273f34c91f71b7fc289. 10.1371/currents.outbreaks.6844c80ff9f5b273f34c91f71b7fc289 [DOI] [PMC free article] [PubMed]
- 24.Pullan S, Dey M. Vaccine hesitancy and anti-vaccination in the time of COVID-19: a Google trends analysis. Vaccine. 2021;39:1877–1881. doi: 10.1016/j.vaccine.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tedeschi SK, Ellrodt J, Stratton J, Santacroce L, Chandler PD, Gravallese EM, Solomon DH. Acceptability of vaccines against preventable infections including coronavirus disease 2019 among patients with rheumatic disease. ACR Open Rheumatol. 2022;4:3–7. doi: 10.1002/acr2.11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be made available upon reasonable request.


