Abstract
Introduction/Aims
Trials incorporating placebo‐to‐active treatment crossover are encouraged in fatal conditions like amyotrophic lateral sclerosis (ALS) but may underestimate active treatment survival benefit. Here, we apply methods for modeling survival without crossover, including the rank‐preserving structural failure time model (RPSFTM), to data from the CENTAUR trial of sodium phenylbutyrate and taurursodiol (PB and TURSO) in ALS incorporating both randomized placebo‐controlled and open‐label extension (OLE) phases.
Methods
Intent‐to‐treat (ITT) and RPSFTM survival analyses were performed with final data at a July 2020 cutoff date. Analyses of subgroups based on randomized treatment and OLE phase participation were also performed.
Results
Hazard ratios (95% confidence intervals) of death for PB and TURSO versus participants initially on placebo were 0.57 (0.35–0.92) on ITT analysis and 0.39 (0.17–0.88) in the primary on‐treatment RPSFTM analysis (p = .023). Median ITT survival duration for PB and TURSO (25.8 mo) was 6.9 mo longer than placebo (18.9 mo) on ITT analysis and 10.6 mo longer than the median RPSFTM‐adjusted survival duration for placebo (15.2 mo). Median survival duration was 18.8 mo longer in the PB and TURSO–randomized subgroup who continued into the OLE phase versus the placebo‐randomized subgroup who did not continue into the OLE phase (p < .0001), although OLE phase selection bias may have potentially confounded these results.
Discussion
Similar to the prespecified ITT analysis, post hoc analyses adjusting for treatment crossover in CENTAUR showed a significant survival benefit for PB and TURSO. Such methods may provide clinical context for observed survival outcomes in future ALS crossover trials.
Keywords: amyotrophic lateral sclerosis, clinical trial, crossover trial design, sodium phenylbutyrate and taurursodiol, survival
Intent‐to‐treat (ITT) analyses of trials incorporating placebo‐to‐active treatment crossover may underestimate the survival benefit of investigational therapies. In the CENTAUR trial of sodium phenylbutyrate and taurursodiol (PB and TURSO) in ALS incorporating an open‐label extension phase, median ITT survival duration for PB and TURSO was 6.9 months longer than placebo on ITT analysis and 10.6 months longer than placebo in the primary on‐treatment rank‐preserving structural failure time model (RPSFTM) analysis, which modeled survival in the absence of treatment crossover.
1. INTRODUCTION
Of the two approved disease‐modifying therapies for amyotrophic lateral sclerosis (ALS), only riluzole has been shown to improve survival (specifically, tracheostomy‐free survival) in clinical trials. 1 , 2 Recently, an orally administered, fixed‐dose coformulation of sodium phenylbutyrate and taurursodiol (PB and TURSO) was shown to significantly slow functional decline and to prolong overall survival in people with ALS in the phase 2 CENTAUR trial, which encompassed both randomized placebo‐controlled (NCT03127514) 3 and open‐label extension (OLE) (NCT03488524) 4 phases. Median survival duration was 6.5 mo longer in those originally randomized to PB and TURSO versus placebo in an interim intent‐to‐treat (ITT) analysis spanning both the randomized and OLE phases, 4 but the crossover design of trials like CENTAUR is expected to underestimate the effect of active treatment on overall survival compared with a true placebo. 5 , 6 , 7 , 8 While incorporating an option for receiving active treatment in an OLE phase is of critical importance in the design of trials for a rapidly progressive and fatal disease like ALS, such a design may lead to underestimation of the clinical effect of investigational therapies when comparing earlier initiation of treatment to later initiation, with subsequent effects on both clinical decision‐making and health technology assessments. 6 , 9 , 10
Analyses evaluating crossover trial subgroups based on both originally randomized treatment and OLE phase participation provide a simple approach that may provide insight into the potential effect of treatment switching in the control group; however, the results of such analyses are likely biased by differences in prognosis between the OLE phase and non–OLE phase subgroups. 6 , 11 More complex statistical methods for modeling survival benefit in the absence of treatment crossover exist that theoretically eliminate such bias, including rank‐preserving structural failure time models (RPSFTMs). 11 These models are used in the analysis of oncology trials, which frequently incorporate a crossover design based on ethical considerations, and are generally regarded as appropriate methods for assessing the potential effect of treatment crossover on clinical trial survival estimates. 5 , 6
In this article, we present the final ITT analyses of survival data from CENTAUR and apply the aforementioned analytic methods to these data post hoc to provide insight into the potential effect of treatment crossover in the placebo group on the estimated survival effect of PB and TURSO.
2. METHODS
2.1. CENTAUR trial
CENTAUR was conducted at 25 Northeast ALS Consortium centers. Protocol approval for both the randomized and OLE phases was provided by a central institutional review board, the Partners Human Research Committee, for all trial sites. Participants provided written informed consent before entering each trial phase. Detailed methods of both trial phases have been published. 3 , 4 Briefly, adults with definite ALS (revised El Escorial criteria) who were ≤18 mo from symptom onset were randomized 2:1 to receive daily PB and TURSO or placebo by mouth or feeding tube. Participants completing the randomized phase were eligible to enroll in the OLE phase and receive daily PB and TURSO for up to 40 mo total. Continuation of a stable dose of riluzole at baseline was permitted, as was initiation or continuation of edaravone during both phases of the trial.
2.2. ITT survival analyses
Results from an interim ITT survival analysis performed at a cutoff date of July 20, 2020 (longest follow‐up, 35 mo after randomization) have been previously reported. 4 Updated analyses of the finalized dataset from CENTAUR incorporating updated participant vital status information as of the prior interim ITT analysis, at both the prior July 2020 cutoff date and the date of the final OLE phase participant visit (March 1, 2021), are reported here (Supporting Information Figure S1).
The prespecified ITT survival analyses compared time to death (all‐cause mortality) between participants originally randomized to PB and TURSO versus placebo (Supporting Information Figure S1). Methods for participant vital status determination have been described. 4 For the post hoc subgroup analysis, the study population was broken into four subgroups based on a) whether participants were originally randomized to PB and TURSO versus placebo and b) whether participants enrolled in the OLE phase or not (Supporting Information Figure S1). For both the prespecified ITT survival analysis and post hoc subgroup survival analysis, the hazard ratio (HR) of death in the group originally randomized to PB and TURSO versus the group originally randomized to placebo was estimated using a Cox proportional hazards model with covariates of age at randomization, pre‐baseline Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS‐R) slope, and baseline ALSFRS‐R total score. Median survival durations and 95% confidence intervals (CIs) were estimated from Kaplan–Meier plots; tests were declared significant if two‐tailed p values were ≤ .05.
2.3. RPSFTM analyses
For consistency with the primary analyses, the entire randomized population was used for the RPSFTM analyses. The RPSFTM end point was defined in the same way as for the primary analyses, using all‐cause mortality (Supporting Information Figure S1).
In RPSFTM analyses, the crossover treatment effect duration can be assumed to last from the first dose of active drug until death or censoring (“treatment group” approach) or encompass only the days the participant received active drug (“on‐treatment” approach). The on‐treatment approach was used as the primary analysis herein, with the treatment group approach used as a sensitivity analysis. For the on‐treatment approach, the number of days on PB and TURSO was defined as the date of last dose (in the randomized or OLE phase) minus the date of first dose (in the randomized phase or OLE phase) +1.
An acceleration factor (AF) corresponding with the extent by which active treatment either extended or decreased survival time 10 was determined using G‐estimation and was then used to adjust the survival estimate for participants in the placebo arm who switched to PB and TURSO; additional details are provided in Supporting Information Appendix S1. Given the potential for informative censoring bias, multiple recensoring approaches were applied as sensitivity analyses (see Supporting Information Appendix S1 for details).
Kaplan–Meier plots were produced for the adjusted survival in the arm originally randomized to placebo using both on‐treatment and treatment group approaches. Adjusted survival estimates in the group originally randomized to placebo were compared with observed survival in the group originally randomized to PB and TURSO from the prespecified ITT analysis (Supporting Information Figure S1). As for the primary analyses, HRs were estimated using a Cox proportional hazards model with covariates of age at randomization, pre‐baseline ALSFRS‐R slope, and baseline ALSFRS‐R total score. Confidence intervals and p values were based on the ITT p value.
3. RESULTS
3.1. Participants
A total of 137 participants were randomized in CENTAUR (PB and TURSO, n = 89; placebo, n = 48); 90 of 98 eligible participants continued into the OLE phase, including 71% (34/48) of participants originally randomized to placebo. In this final analysis, vital status was obtainable for all but one participant randomized in CENTAUR; this participant was censored at the date of last follow‐up visit. Additional participant disposition as well as baseline data have been previously published. 4
3.2. ITT analyses
In the final overall survival analysis encompassing all randomized participants at the July 2020 cutoff date, median (95% CI) survival duration was 25.8 mo (19.0 mo–not reached [NR]) in the group originally randomized to PB and TURSO and 18.9 (13.5–28.7) mo in the group originally randomized to placebo (6.9‐mo difference), with an HR of 0.57 (Figure 1); mean PB and TURSO exposure durations were 10.2 mo in the group originally randomized to PB and TURSO and 4.6 mo in the group originally randomized to placebo (all in the OLE phase). Results of the survival analysis at the March 2021 cutoff date were concordant, showing a significantly lower hazard of death and longer median survival duration in the group originally randomized to PB and TURSO (Supporting Information Figure S2).
FIGURE 1.
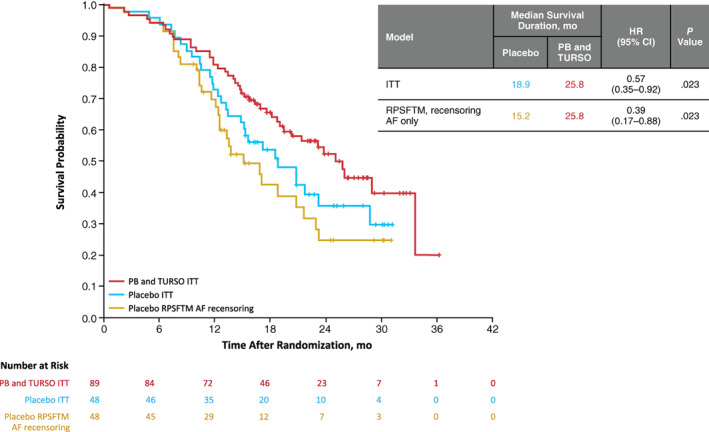
Kaplan–Meier analyses: Prespecified ITT survival analysis (red and blue lines) and rank‐preserving structural failure time model–adjusted survival (yellow line, recensoring acceleration factor only; on‐treatment approach). All analyses incorporate final data from the July 20, 2020, cutoff date, including updated participant vital status information as of the prior published interim ITT analysis at this cutoff date 4
3.3. RPSFTM analyses
On‐treatment RPSFTM analyses of data were performed through the July 2020 cutoff date and applying recensoring of the AF. Compared with the observed estimate of 25.8 mo for the group originally randomized to PB and TURSO in the prespecified ITT analysis, RPSFTM‐adjusted median survival duration was 15.2 mo in the group originally randomized to placebo (difference of 10.6 mo); HR was 0.39 (Figure 1). Similar results were obtained with full recensoring, with an HR of 0.44 (Supporting Information Figure S3), consistent with a beneficial effect (AF <1) of PB and TURSO treatment on overall survival. AF could not be estimated in assessments of on‐treatment RPSFTM without applying recensoring. Sensitivity analyses using the treatment group RPSFTM yielded similar results to the on‐treatment analyses (Supporting Information Figure S4). The same on‐treatment and treatment group RPSFTM analyses were performed on data through the March 2021 cutoff date and also yielded consistent results (Supporting Information Figures S2 and S5, respectively).
3.4. Post hoc subgroup analysis
Results of the post hoc survival analysis of subgroups based on originally randomized treatment and OLE phase participation through the July 2020 cutoff date are shown in Figure 2. Median survival duration decreased and hazard of death increased with decreasing PB and TURSO exposure duration (all p ≤ .01 compared with the subgroup of participants who were originally randomized to PB and TURSO and continued into the OLE phase). Median survival duration was 18.8 mo longer in the group who were originally randomized to PB and TURSO and continued into the OLE phase (33.6 mo) than in the group who were originally randomized to placebo and did not cross over to active treatment in the OLE phase (14.8 mo; p < .0001). Among the other subgroups, median survival duration was longer in the group who were originally randomized to placebo and continued into the OLE phase, who had a longer mean PB and TURSO exposure duration, compared with the group who were originally randomized to PB and TURSO but did not continue into the OLE phase. Subgroup analysis at the March 2021 cutoff date yielded similar results (Supporting Information Figure S6).
FIGURE 2.
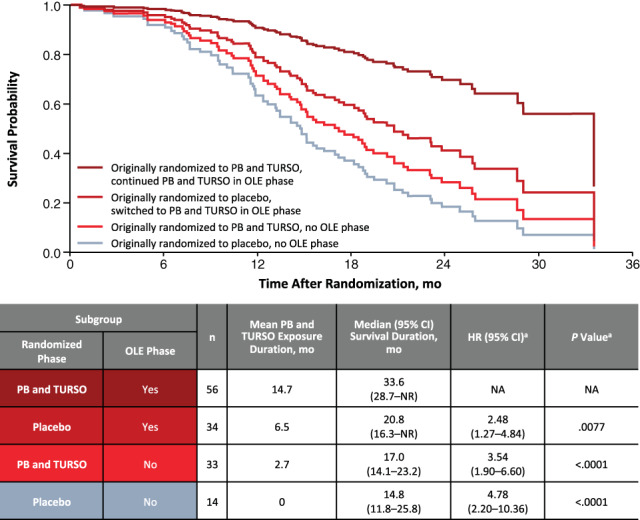
Cox proportional hazards analysis of time to death: Subgroups based on originally randomized treatment and OLE phase participation (July 2020 cutoff date). aCompared with subgroup originally randomized to sodium phenylbutyrate and taurursodiol that entered the OLE phase
4. DISCUSSION
In our RPSFTM analyses of data from CENTAUR, statistical methods that adjust for treatment crossover in the group originally randomized to placebo suggested a greater survival benefit with PB and TURSO use than seen in the ITT analysis.
Post hoc assessment of subgroups from CENTAUR based on randomization group as well as enrollment in the OLE phase demonstrated that earlier and longer exposure to PB and TURSO was associated with longer survival estimates. The finding of longer median survival duration in the subgroup of participants who were originally randomized to placebo and crossed over to active treatment in the OLE phase versus the subgroup who were originally randomized to PB and TURSO but did not continue into the OLE phase further supports an association between PB and TURSO exposure duration and survival outcome in CENTAUR.
It is important to note that the post hoc subgroup analysis applied herein has limitations as the subgroups are small, and potential confounding differences among the groups were not controlled for, although covariates of age at randomization, pre‐baseline ALSFRS‐R slope, and baseline ALSFRS‐R total score attempted to control for some of this bias. In the RPSFTM analysis, it was not possible to estimate the AF for the RPSFTM without recensoring using the on‐treatment duration of effect assumption. No factor could be selected that balanced the counterfactual survival (ie, the model‐estimated survival time in the absence of PB and TURSO) without recensoring between the treatment arms. It may be due to a prognostic imbalance at baseline between randomized arms, although this seems unlikely as baseline characteristics were generally well balanced and the AF could be estimated from other models. Alternatively, it may be that the on‐treatment duration of effect assumption is not suitable for this study, but this was judged to be a more plausible assumption than the treatment group approach. Similar results were seen for the analyses using both duration of effect assumptions, suggesting that varying this assumption does not have a large impact.
Another important point about the RPSFTM method is that it assumes a common treatment effect (ie, exposure‐response is the same, no matter when the treatment is received). 11 This assumption may be unreliable in degenerative conditions such as ALS, although the time between randomization and crossover in CENTAUR was relatively short (only 6 mo). In addition, the subgroup of participants who were originally randomized to placebo and continued into the OLE phase had a lower risk of death and longer median survival than the subgroup who were originally randomized to active treatment and discontinued after the randomized phase, indicating that exposure to drug and not current severity of disease was more important to survival. Finally, PB and TURSO targets neuronal death, which is expected to be relevant at all stages of disease represented in the trial, although again, it cannot be known for certain whether treatment effects at earlier versus later time points might differ. Other methods such as inverse probability of censoring weighting and two‐stage models are often used in oncology to adjust overall survival for switching and were considered here; however, these methods are not suitable when most participants switch, as was the case in the CENTAUR trial, in which only three of the placebo‐randomized participants who were eligible to enroll in the OLE phase did not do so.
In conclusion, ALS clinical trials can gather robust survival data while incorporating study design elements, such as OLE phases, that are critical for facilitating access to investigational therapies for people with ALS. However, the inherent inability to sustain a true placebo group long enough to assess survival in such studies may lead to underestimation of the clinical effect of the therapies under investigation when having to resort to comparing earlier initiation of treatment to later initiation. By adjusting survival estimates in the presence of treatment switching, methods such as RPSFTM and subgroup analyses may provide additional clinical context beyond the observed survival outcomes in ALS trials incorporating this critical crossover design that may be informative for patients and other stakeholders. In post hoc analyses of the CENTAUR trial, these two methods yielded a 10.6‐ and 18.8‐mo adjusted survival benefit of PB and TURSO, respectively. Additional data from the ongoing phase 3 PHOENIX trial (NCT05021536) and clinical experience will provide further information regarding the effect of PB and TURSO on survival in people with ALS.
AUTHOR CONTRIBUTIONS
Dr Paganoni had full access to the trial data and takes full responsibility for the integrity of the data and the accuracy of the data analysis. Dr Paganoni, Ms Watkins, Mr Cawson, and Drs Timmons, Manuel, and Cudkowicz were involved in the conceptualization of the research described in the article. Ms Watkins, Mr Cawson, Drs Hendrix and Dickson, and Mr Knowlton curated and analyzed the data; oversaw the methodology and provided software for the analyses described in the article; and contributed to the validation and visualization of the analysis results. Drs Paganoni and Cudkowicz contributed to data acquisition in CENTAUR and provided oversight and leadership for the planning and execution of the research described in the article. Drs Timmons and Manuel provided project administration oversight. Ms Watkins, Mr Cawson, Drs Hendrix and Dickson, Mr Knowlton, and Drs Timmons and Manuel contributed to provision of resources, including study materials and analysis tools. Ms Watkins, Mr Cawson, and Dr Timmons contributed to the first‐draft development of the article, and all authors critically reviewed and revised the article at each stage of development and approved the final draft for submission.
CONFLICTS OF INTEREST
Sabrina Paganoni reports research grants from Amylyx Pharmaceuticals, Revalesio Corporation, Alector Therapeutics, UCB, Biohaven Pharmaceuticals, Clene Nanomedicine, Prilenia Therapeutics, Seelos Therapeutics, The ALS Association, the American Academy of Neurology, the Centers for Disease Control, ALS Finding a Cure®, the Salah Foundation, the Spastic Paraplegia Foundation, the Muscular Dystrophy Association, I AM ALS, Tambourine, Target ALS, Columbia University, and the Cullen Education and Research Fund, and is a site principle investigator for studies funded by Alector Therapeutics, Cytokinetics, and Novus (formerly Anelixis) Therapeutics. Dr Paganoni also reports institutional consulting agreements with Amylyx Pharmaceuticals, Frequency Therapeutics, and SOLA Biosciences; personal consulting agreements with Cytokinetics, Arrowhead Pharmaceuticals, and Orthogonal Neuroscience; and honoraria from Medscape. Claire Watkins and Matthew Cawson report fees via the Maple Health Group from Amylyx Pharmaceuticals for conducting some of the analyses described in the submitted work. Suzanne Hendrix, Samuel P. Dixon, and Newman Knowlton are employees of Pentara Corporation. Jamie Timmons and Machelle Manuel are full‐time employees of Amylyx Pharmaceuticals. Merit Cudkowicz reports consulting fees from Faze, Regeneron, AB Sciences, Avexis, MT Pharma, Revalasio, Cytokinetics, Disarm, ALS Pharma, Immunity Pharma, Wave, Sunovian, Transposon, Quralis, Helixsmith, Locust Walk, and RRD and is a board member for Praxis, all outside the submitted work.
Abbreviations
- AF
acceleration factor
- ALS
amyotrophic lateral sclerosis
- ALSFRS‐R
Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised
- CI
confidence interval
- HR
hazard ratio
- ITT
intent‐to‐treat
- NA
not applicable
- NR
not reached
- OLE
open‐label extension
- PB and TURSO
sodium phenylbutyrate and taurursodiol
- RPSFTM
rank‐preserving structural failure time model
ETHICAL PUBLICATION STATEMENT
The authors confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1 Schematic of survival analyses conducted on CENTAUR dataset. Time to all‐cause death was assessed over the periods spanning randomization through each cutoff date and compared among the groups shown for each analysis (table). The intent‐to‐treat (ITT) analysis was prespecified, while the other analyses were performed post hoc. aThe ITT population consisted of all participants randomized in CENTAUR who received ≥1 dose of trial drug in the randomized phase. OLE, open‐label extension; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S2 Kaplan–Meier analyses: prespecified intent‐to‐treat (ITT) survival analysis (red and blue lines) and rank‐preserving structural failure time model (RPSFTM)–adjusted survival in the originally randomized placebo group (yellow line, recensoring acceleration factor [AF] only; purple line, full recensoring; on‐treatment approach). All analyses incorporate matured data from the March 1, 2021, cutoff date coinciding with the final participant visit in CENTAUR. The AF used to adjust survival for switching could not be estimated in the on‐treatment RPSFTM without applying recensoring. HR, hazard ratio; PB and TURSO, sodium phenylbutyrate and taurursodiol.
Figure S3 Kaplan–Meier analyses: prespecified intent‐to‐treat (ITT) survival analysis (red and blue lines) and rank‐preserving structural failure time model (RPSFTM)–adjusted survival (purple line, full recensoring; on‐treatment approach). All analyses incorporate final data from the July 20, 2020, cutoff date, including updated participant vital status information as of the prior published interim ITT analysis at this cutoff date [Paganoni S et al. Muscle Nerve. 2021;63(1):31–39]. The acceleration factor used to adjust survival for switching could not be estimated in the on‐treatment RPSFTM without applying recensoring. HR, hazard ratio; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S4 Kaplan–Meier analysis: prespecified intent‐to‐treat survival analysis (red and blue lines) and rank‐preserving structural failure time model–adjusted survival (green line, no recensoring; yellow line, recensoring acceleration factor only; purple line, full recensoring; treatment group approach). All analyses incorporate final data from the July 20, 2020, cutoff date. AF, acceleration factor; HR, hazard ratio; ITT, intent‐to‐treat; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S5 Kaplan–Meier analysis: prespecified intent‐to‐treat survival analysis (red and blue lines) and rank‐preserving structural failure time model–adjusted survival (green line, no recensoring; yellow line, recensoring acceleration factor only; purple line, full recensoring; treatment group approach). All analyses incorporate final data from the March 1, 2021, cutoff date. AF, acceleration factor; HR, hazard ratio; ITT, intent‐to‐treat; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S6 Cox proportional hazards analysis of time to death: subgroups based on originally randomized treatment and open‐label extension (OLE) phase participation (March 2021 cutoff date). aCompared with subgroup originally randomized to sodium phenylbutyrate and taurursodiol that entered the OLE phase. HR, hazard ratio; NA, not applicable; NR, not reached; PB and TURSO, sodium phenylbutyrate and taurursodiol.
Appendix S1 Supporting information
ACKNOWLEDGMENTS
Funding for the CENTAUR trial was provided by Amylyx Pharmaceuticals, Inc., ALS Finding a Cure®, and The ALS Association. Funding for the post hoc RPSFTM analyses, which were conducted by the Maple Health Group, was provided by Amylyx. The authors thank the individuals who participated in the randomized and open‐label extension phases of the CENTAUR trial as well as their caregivers and families, and the CENTAUR investigators and coordination center and trial site staff. This analysis was funded by Amylyx Pharmaceuticals, Inc. Theresa Leichner, PhD, and Lara Primak, MD, of PRECISIONscientia provided medical writing assistance with the development and revision of the manuscript under the direction of the authors, with financial support from Amylyx and in compliance with international Good Publication Practice guidelines.
Paganoni S, Watkins C, Cawson M, et al. Survival analyses from the CENTAUR trial in amyotrophic lateral sclerosis: Evaluating the impact of treatment crossover on outcomes. Muscle & Nerve. 2022;66(2):136‐141. doi: 10.1002/mus.27569
Portions of this manuscript were presented at the 2021 Annual Northeast Amyotrophic Lateral Sclerosis Consortium Meeting and the 2021 International Symposium on ALS/MND.
Funding information ALS Finding a Cure; Amylyx Pharmaceuticals, Inc.; The ALS Association
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Andrews JA, Jackson CE, Heiman‐Patterson TD, Bettica P, Brooks BR, Pioro EP. Real‐world evidence of riluzole effectiveness in treating amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2020;21(7–8):509‐518. [DOI] [PubMed] [Google Scholar]
- 2. Schultz J. Disease‐modifying treatment of amyotrophic lateral sclerosis. Am J Manag Care. 2018;24(15 Suppl):S327‐S335. [PubMed] [Google Scholar]
- 3. Paganoni S, Macklin EA, Hendrix S, et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N Engl J Med. 2020;383(10):919‐930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paganoni S, Hendrix S, Dickson SP, et al. Long‐term survival of participants in the CENTAUR trial of sodium phenylbutyrate‐taurursodiol in amyotrophic lateral sclerosis. Muscle Nerve. 2021;63(1):31‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ishak KJ, Proskorovsky I, Korytowsky B, Sandin R, Faivre S, Valle J. Methods for adjusting for bias due to crossover in oncology trials. Pharmacoeconomics. 2014;32(6):533‐546. [DOI] [PubMed] [Google Scholar]
- 6. Jönsson L, Sandin R, Ekman M, et al. Analyzing overall survival in randomized controlled trials with crossover and implications for economic evaluation. Value Health. 2014;17(6):707‐713. [DOI] [PubMed] [Google Scholar]
- 7. Liu‐Seifert H, Andersen SW, Lipkovich I, Holdridge KC, Siemers E. A novel approach to delayed‐start analyses for demonstrating disease‐modifying effects in Alzheimer's disease. PLoS One. 2015;10(3):e0119632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spineli LM, Jenz E, Großhennig A, Koch A. Critical appraisal of arguments for the delayed‐start design proposed as alternative to the parallel‐group randomized clinical trial design in the field of rare disease. Orphanet J Rare Dis. 2017;12(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Latimer NR, White IR, Abrams KR, Siebert U. Causal inference for long‐term survival in randomised trials with treatment switching: should re‐censoring be applied when estimating counterfactual survival times? Stat Methods Med Res. 2019;28(8):2475‐2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sullivan TR, Latimer NR, Gray J, Sorich MJ, Salter AB, Karnon J. Adjusting for treatment switching in oncology trials: a systematic review and recommendations for reporting. Value Health. 2020;23(3):388‐396. [DOI] [PubMed] [Google Scholar]
- 11. Latimer NR, Abrams KR. NICE DSU technical support document 16: adjusting survival time estimates in the presence of treatment switching. Vol 16. London: National Institute for Health and Care Excellence (NICE); 2014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Schematic of survival analyses conducted on CENTAUR dataset. Time to all‐cause death was assessed over the periods spanning randomization through each cutoff date and compared among the groups shown for each analysis (table). The intent‐to‐treat (ITT) analysis was prespecified, while the other analyses were performed post hoc. aThe ITT population consisted of all participants randomized in CENTAUR who received ≥1 dose of trial drug in the randomized phase. OLE, open‐label extension; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S2 Kaplan–Meier analyses: prespecified intent‐to‐treat (ITT) survival analysis (red and blue lines) and rank‐preserving structural failure time model (RPSFTM)–adjusted survival in the originally randomized placebo group (yellow line, recensoring acceleration factor [AF] only; purple line, full recensoring; on‐treatment approach). All analyses incorporate matured data from the March 1, 2021, cutoff date coinciding with the final participant visit in CENTAUR. The AF used to adjust survival for switching could not be estimated in the on‐treatment RPSFTM without applying recensoring. HR, hazard ratio; PB and TURSO, sodium phenylbutyrate and taurursodiol.
Figure S3 Kaplan–Meier analyses: prespecified intent‐to‐treat (ITT) survival analysis (red and blue lines) and rank‐preserving structural failure time model (RPSFTM)–adjusted survival (purple line, full recensoring; on‐treatment approach). All analyses incorporate final data from the July 20, 2020, cutoff date, including updated participant vital status information as of the prior published interim ITT analysis at this cutoff date [Paganoni S et al. Muscle Nerve. 2021;63(1):31–39]. The acceleration factor used to adjust survival for switching could not be estimated in the on‐treatment RPSFTM without applying recensoring. HR, hazard ratio; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S4 Kaplan–Meier analysis: prespecified intent‐to‐treat survival analysis (red and blue lines) and rank‐preserving structural failure time model–adjusted survival (green line, no recensoring; yellow line, recensoring acceleration factor only; purple line, full recensoring; treatment group approach). All analyses incorporate final data from the July 20, 2020, cutoff date. AF, acceleration factor; HR, hazard ratio; ITT, intent‐to‐treat; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S5 Kaplan–Meier analysis: prespecified intent‐to‐treat survival analysis (red and blue lines) and rank‐preserving structural failure time model–adjusted survival (green line, no recensoring; yellow line, recensoring acceleration factor only; purple line, full recensoring; treatment group approach). All analyses incorporate final data from the March 1, 2021, cutoff date. AF, acceleration factor; HR, hazard ratio; ITT, intent‐to‐treat; PB and TURSO, sodium phenylbutyrate and taurursodiol; RPSFTM, rank‐preserving structural failure time model.
Figure S6 Cox proportional hazards analysis of time to death: subgroups based on originally randomized treatment and open‐label extension (OLE) phase participation (March 2021 cutoff date). aCompared with subgroup originally randomized to sodium phenylbutyrate and taurursodiol that entered the OLE phase. HR, hazard ratio; NA, not applicable; NR, not reached; PB and TURSO, sodium phenylbutyrate and taurursodiol.
Appendix S1 Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


