Abstract
Objectives
Multidisciplinary teams in cancer care are increasingly using information and communication technology (ICT), hospital health information system (HIS) functionalities and ICT-driven care components. We aimed to explore the use of these tools in multidisciplinary team meetings (MTMs) and to identify the critical challenges posed by their adoption based on the perspective of professionals representatives from European scientific societies.
Design
This qualitative study used discussion of cases and focus group technique to generate data. Thematic analysis was applied.
Setting
Healthcare professionals working in a multidisciplinary cancer care environment.
Participants
Selection of informants was carried out by European scientific societies in accordance with professionals’ degree of experience in adopting the implementation of ICT and from different health systems.
Results
Professionals representatives of 9 European scientific societies were involved. Up to 10 ICTs, HIS functionalities and care components are embedded in the informational and decision-making processes along three stages of MTMs. ICTs play a key role in opening MTMs to other institutions (eg, by means of molecular tumour boards) and information types (eg, patient-reported outcome measures), and in contributing to the internal efficiency of teams. While ICTs and care components have their own challenges, the information technology context is characterised by the massive generation of unstructured data, the lack of interoperability between systems from different hospitals and HIS that are conceived to store and classify information rather than to work with it.
Conclusions
The emergence of an MTM model that is better integrated in the wider health system context and incorporates inputs from patients and support systems make traditional meetings more dynamic and interconnected. Although these changes signal a second transition in the development process of multidisciplinary teams, they occur in a context marked by clear gaps between the information and management needs of MTMs and the adequacy of current HIS.
Keywords: oncology, health services administration & management, information technology, telemedicine
Strengths and limitations of this study.
The paper proposes an exploration of the mostly adopted information and communication technologies (ICTs), hospital health information system functionalities and ICT-driven care components in multidisciplinary team meetings (MTMs).
A qualitative study was conducted based on key informants from different European scientific societies and health systems.
Key informants were experienced in adopting the implementation of ICT in MTMs, and this was useful for both case presentation (including unsuccessful practices) and focus group discussion.
Owing to the explorative nature of the study, it was not possible to capture all ICTs and care components being used in MTMs and this way achieve data saturation.
Introduction
Since the 1990s, multidisciplinary teams (MDTs) for cancer care have improved their internal organisation, increasing the representativeness of the team by including more roles and broadening care objectives and scope of practice to new areas of care (eg, survivorship care).1 Although there are pronounced organisational and financial differences between MDTs from different European health systems,2 all MDTs are characterised by the central role of the multidisciplinary team meeting (MTM)—also referred as tumour board or multidisciplinary cancer conference—as the main decision-making body.3 These meetings represent a widely recognised standard of care, including in different accreditation and quality systems.4–7
The use of information and communication technologies (ICTs) have taken off in the 21st century, facilitating new modes of MDT interaction and streamlining information management processes.8 In fact, the potential to transform multidisciplinary cancer care extends beyond typical ICT functionalities such as virtual MTMs and telehealth, encompassing the integration of other care components such as patient-reported outcome measures (PROMs) and clinical decision support systems (CDSS) into hospitals’ ICT and health information systems (HIS). The adoption of ehealth practice is generally modest and uneven between different European health systems, and unsuccessful experiences are not unheard of; however, the qualitative leap in the use of ICTs—clearly accelerated by the COVID-19 pandemic9 10—and associated care components raises the question of whether MDTs are undergoing a second transition.
The European Commission-supported Innovative Partnership for Action Against Cancer (iPAAC) defined as a priority the issue of how ICTs affect the daily work of cancer MDTs, an ambitious endeavour that was tackled in collaboration with the European scientific societies. In this study, we explored the set of ICTs, HIS-based functionalities and associated care components used by MTMs in order to identify the critical challenges posed by their adoption based on the perspective of professionals representatives from European scientific societies.
Methods
Study design and setting
Health professionals’ perspectives on the use of ICTs, HIS and associated care components in cancer MTMs were analysed by qualitative methodology. A multidisciplinary European workshop, lasting approximately 5 hours, was organised on 5 July 2019 in a neutral setting (European Cancer Organisation (ECCO) headquarters in Brussels). The workshop was divided in two phases. In the first, each professional presented a prepared case study based on their local experience and healthcare system. The contrasts sparked discussions about the adoption and practices of ICT-led informational and clinical decision-making processes embedded in MTMs. Second, focus groups were used to explore the opinions and normative systems through group interactions11 from the perspective of each medical discipline, which brought to light conceptual-based reflections and knowledge about the relevance of the different ICTs, HIS functionalities and ICT-driven care components.
Selection of informants and sampling strategy
The workshop was co-organised between the Catalan Institute of Oncology and ECCO within the framework of the iPAAC Joint Action. ECCO played a gatekeeper role in the selection of key informants, sending a letter of invitation prepared by the researchers to different European scientific societies and explaining the reasons for the study. For selection of informants and composition of the purposive sample, informants were designated by the scientific societies according to four inclusion criteria: (1) representing the diagnosis and treatment perspectives and including other relevant issues in cancer care (eg, oncogeriatrics); (2) experienced in leading and/or adopting the implementation of ICT; (3) working in a multidisciplinary cancer care environment; and (4) from different healthcare areas and European health systems. The exclusion criterion, emphasised by ECCO when contacting the different societies, consisted of avoiding the participation of experts in medical technologies or ICTs exclusively from a technical point of view. Clinical reasoning on ICTs rather than focusing on technologies themselves was the critical aspect of the selection. Guidance on group size is common and seldom goes beyond a minimum of 4 and a maximum of 12,12 but we restricted this number to 10 in order to make it manageable. Nine professionals from different European scientific societies and from four health systems, including the Organisation of European Cancer Institutes, were finally enrolled (table 1). They were included as coauthors of this study.
Table 1.
Affiliations of the nine professionals that took part in the workshop
| Organisation | Country | Profession | Sex | Years of experience |
| European Society of Radiology | Italy | Radiologist | Male | 33 |
| European Association of Nuclear Medicine | Belgium | Nuclear medicine physician | Female | 9 |
| European Oncology Nursing Society | Belgium | Oncology nursing | Male | 21 |
| European Society of Oncology Pharmacy | Croatia | Clinical pharmacy specialist | Male | 6 |
| International Society of Geriatric Oncology | Belgium | Medical oncologist | Female | 15 |
| Organisation of European Cancer Institutes | Pan-European | Manager of international health organisations | Male | 45 |
| European Society for Radiotherapy & Oncology | Italy | Radiation oncologist | Male | n/a |
| European Society of Medical Oncology | Spain | Medical oncologist | Male | 22 |
| European Society of Gynaecological Oncology | Spain | Gynaecologist and obstetrician | Male | 30 |
Analysis
Two researchers conducted the meeting, with one acting as moderator (JP) and the other as observer (CC-O). A sheet containing information about the study goals and a consent form were handed out before starting. The researchers (CC-O, JP) took field notes during the case study presentations. Spontaneous interaction was encouraged during the focus group session, which was recorded and transcribed verbatim. Researchers checked for consistency between the recording and text and conducted the subsequent analysis. Four issues (corresponding to MTM stages) were used to organise the discussion: patient data collection and accessibility, case presentation, results and implications of MTMs discussions, and virtual MTMs (box 1).
Box 1. Cancer multidisciplinary team meetings (MTMs) and information and communication technologies: focus group script.
Data collection and accessibility
How are the patients’ lists drawn up?
How is patient information collected (sources; use of EHRs)?
Are non-tumour-specific issues (such as psico-oncology or oncogeriatrics) captured? How?
Is the case presentation structured (eg, on the basis of a template)? Is it electronically linked to the hospital HIS or prepared on a separate file?
Patient case presentation and decision-making
How is the case presented? What information is it based on?
Are pretreatment digitised images required in the MTMs? What quality criteria are used, if any, and what display problems have you encountered? What interoperability exists with other institutions and information technology systems integration (ie, degree of standardisation)?
What are the technological conditions (eg, high-definition projector; double-screen; PCs in the room)?
Describe the use of PROMs/CDSS (ie, layers of information like protocols; technology at the front line).
Results and implications of MTMs discussions
Are the minutes of the MTM available and accessible?
Are decisions recorded on the EHR?
How are medical appointments organised?
How team results are assessed using HIS (eg, toxicity, QoL issues; MTMs information as output)?
Are MTM decisions and clinical outcomes (real-world data) connected to/feeding AI systems?
Virtual MTMs
What is your experience with virtual MTMs? What challenges are associated with them?
Types: ‘expert’ and ‘non-expert’ teams; communication between expert teams; etc.
How virtual MTMs are organised and implemented (engagement of dispersed members, specialists, GPs)?
Interoperability, privacy and confidentiality of patient data issues
How reliable is the technology? What difficulties exist, if any, in using technology outside a single organisation (eg, virtual consultation of tests)?
AI, Artificial Intelligence; CDSS, clinical decision support system; EHR, electronic health record; GP, General Practitioner; HIS, health information system; PC, personal computer; PROM, patient-reported outcome measure; QoL, quality of life.
To analyse the data, we applied thematic analysis criteria, which emphasise the meaning of the text and interpret its thematic content.13 14 We read through the transcript to identify general themes and specific categories within the themes, ensuring interpreter consensus. Only one researcher coded the data (JP). The research process was inductive, with a constant effort to capture ICTs and other care components related to MTMs, along with their implications and challenges. Figure 1 presents the themes in the form of a coding tree chart. Atlas-ti V.6.2 software15 was used to systematically code and analyse data: all textual data were indexed and co-occurring codes identified. However, the software was used in a limited way to rearrange the data, construct charts and find associations between themes. Preliminary results were discussed among the research team (JP, CC-O, JMB). The initial draft was then widely circulated among workshop participants for final approval. This study was carried out in agreement with the procedures in Consolidated criteria for Reporting Qualitative research.16
Figure 1.
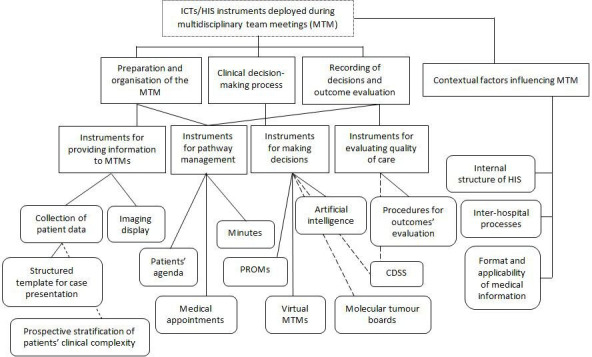
Coding tree for thematic analysis. CDSS, clinical decision support system; HIS, health information system; ICTs, information and communication technologies; PROM, patient-reported outcome measure.
Patient and public involvement
No patient involved.
Results
The results were organised on the basis of four domains that correspond to the three stages of MTM development: (a) preparation and organisation, (b) clinical decision-making process and (c) recording of decisions and outcome evaluation, while the first presented (α) is a transversal domain capturing the contextual perspective. Some quotations from the focus group session are used anonymously in the present paper (box 2).
Box 2. Verbatim examples for each category.
Clinical data and information technology (IT) contextual factors
The electronic health record is an evolution from paper, but it is not an integrated information environment.
We’re slaves to pdfs. We live in the era of medical information in pdf format. The problem is always finding it and using it.
In my hospital there are a lot of systems and quite often they don’t talk to each other. For example, intensive care has a whole different system, so we can’t see what patients have behind if they come from this service. You don’t see the data; you see the summary.
For some CT scans, we cannot radiate the patient again, so we go all the way to retrieve this information, calling the centres, etc. We do not repeat exams for this reason.
For haematology, when we ask for whole body PET but some centres just forget and send it partially. And then you have to repeat tests.
(a) Preparation and organisation of the multidisciplinary team meeting (MTM)
We use a template, a structured framework, since junior doctors are in charge of case presentation.
In the old times we were just sitting next to each other, discussing the files, looking at the images, and someone was moderating the session.
Sometimes we (diagnostician) have to say ‘I’ll give you advice the next day’ and check again at my dedicated work station.
(b) Clinical decision-making process
The patient-reported outcome measures (PROMs) will be important in the future to make decisions in MTMs. With PROMS, the patient is involved in the decision-making process. His/her data is there. It is real-time data.
(On clinical decision support system (CDSS)): These systems appear as a black box. You don’t know what studies and data are in the algorithm. People are afraid because of that.
AI may help but the model is not pressing a button and a decision is made. Interaction between drugs is one of the most evident challenges for a CDSS.
The MTM includes molecular information based on biomarkers such as Ki67 or HER, but which originates in the immunohistochemistry and fluorescence in situ hybridization test, not in the next-generation sequencing. We’re still in the clinical era, but a transition has started.
(c) Recording of decisions and outcome evaluation
From an IT perspective, structured reporting of decisions would be a big change. It’s the clarity that changes, what you don’t find on a free-text report.
ICTs are mainly found before making decisions. Afterwards, they don’t help us: we don’t have much time to arrange the citations, to follow and monitor patients, to look at the results and so on. This could make a difference in optimising the resources.
Sometimes you need something really important for clinical practice and you don’t have it. There is also a lot of unnecessary data.
Clinical data and information technology (IT) contextual factors
Accessible information about cases under discussion in the MTM is essential for agile decision-making. Three elements of the IT context determine the degree of integration, data structuring and standardised collection of medical information.
Hospital HIS: the logic of independent repositories
The informational processes related to MDTs’ activity are largely shaped by the hospital HIS, which is not generally structured around patient care processes but rather around the inputs from different functions or sub-systems of each clinical service (eg, pathology). This means that data collection is performed through independent repositories from which different inputs are extracted in order to draw up a summary of a patient’s case and discuss it in the MTM. Several informants noted the inherent contrast with MDTs, which are cross-sectional by nature and represent care processes in and of themselves (eg, patients with colon cancer), not just a single specialty, service or care episode. Even though electronic health records (EHRs) link different information sources and can be practical enough to use during the MTM, they do not arrange all of the elements relevant to a patient’s diagnosis and treatment in a specific and integrated way.
Free-text and pdf formats and the applicability of medical information
Generally, medical information is not recorded through a single computer system from which it can be extracted or modified in a structured way. Much of the information is in a free-text format, predominantly physician dependent and captured in a pdf, which is difficult to code, use and access. In contrast, if the data records are electronically structured, as demonstrated for breast cancer during the workshop, ICTs/HIS can potentially change how all the available information is collected and visualised during the MTM presentation.
Standardisation of interhospital informational processes
Another factor—which may represent the most time-consuming part of MTM preparation—is obtaining information for patients referred from other hospitals. IT systems from different hospitals are rarely integrated or standardised, so patients are often referred with low-quality images, images that do not meet specific requirements, and even with CD-ROMs, prompting the need for repeating tests. Professionals need to obtain the original information, not just the summary, and they cannot diagnose without downloading the original images in the system to review them properly. The lack of standardisation in the exchange of images causes important delays in decision-making, and in medical specialties applying ionising radiation, this repetition is problematic because it can be harmful to patients’ health. Instead, when different hospitals agree to use a common HIS, and therefore the same EHRs for patients, referring patients does not imply any special obstacles.
Preparation and organisation of the MTM
Multidisciplinary electronic patient agenda and patients’ stratification
Using a multidisciplinary electronic patient agenda to draw up patient lists helps MDTs to better anticipate and rapidly manage case discussions. Professionals wishing to discuss a case on the MTM reserve a time slot for a consultation using the hospital HIS in the same way they would do so for an appointment with any other hospital service. This way, all the professionals can see the list of patients to discuss in real time and then prepare for the meeting accordingly (ie, patients with pending diagnostic tests results may be removed from the list). Nevertheless, informants stressed that such automation is limited in most MDTs, with no computer system used. Typically, the MTM coordinator collects and collates team members’ proposals and then distributes them in the form of a medical chart containing the clinical description of each patient. Professionals also use the electronic agenda to stratify patients into high and low priority cases, distinguishing between cases that should be discussed in depth and those that only require confirmation that the treatment strategy is in line with the guideline. While stratification is informal nowadays, its digitisation would improve efficiency and organisation of the discussion process, cueing the professionals that only need to weigh in on a few cases (eg, reconstructive surgeons, general practitioners, MDT members accessing remotely) on when they should attend.
Checklist and software for patient case presentation
Some MDTs use templates or checklists to present patient cases, while for others the mode of presentation depends on individual professionals or is assumed by junior doctors. The qualitative leap on this point occurs when the hospital HIS (or external software that processes HIS data) is capable of capturing and integrating all the relevant data that MDTs need to make decisions. Professionals can then directly narrate what is shown onscreen, not what is summarised in the medical chart. Structured case presentations have the capacity to improve efficiency, comprehensiveness and rigour during the MTM, for example, by reserving a specific slot to discuss data on the patient’s geriatric situation on the information agenda. However, informants expressed caution about basing the MTM discussion on rigid checklists and computerised categories, since it may limit the individualisation and open discussion of every patient.
Picture archiving and communication system (PACS) and imaging display
The PACS workstation is crucial for medical imaging digitalisation and can be used in combination with a simple software programme to allow MDTs to visualise the images directly on the projector or screen used in the meeting. This greatly facilitates the presentation of images and contributes to synchronising the MDT’s work; however, not all MTMs have this connection, and the ability to interpret nuclear medicine images using PACS is limited.
Clinical decision-making process
Patient-reported outcome measures (PROMs)
Informants believed that PROMs (eg, a symptoms questionnaire completed by patients) help to improve decision-making in MTMs by offering real-time data for discussion, reducing delays and rediscussions. For example, a PROM alert system could warn the MDT that an endometrial cancer patient is oedematous, triggering cancellation of surgery. Some uncertainty existed about whether patients should fill in the PROMs questionnaires alone or with assistance (from a health professional or dedicated software) to help them interpret the questions.
Artificial intelligence and CDSS
Artificial intelligence, especially CDSS, which rely on pre-established clinical algorithms as well as real-world data, provokes conflicting reactions in the sphere of MTMs. While most informants expressed scepticism and misgivings, some have also implemented ‘home-made’ web-based platforms or were willing to experiment and discover their real potential (eg, as a supportive tool indicating patients’ risk of local recurrence). Informants identified three main challenges posed by CDSS. First, CDSS should have safeguards to ensure that decision-making is robust and reproducible. Lack of trustworthiness was foreseen if CDSS propose treatment strategies based on unknown criteria or criteria that may not have been clinically validated by a physician. Second, continuous updates are essential to take into account new scientific evidence and avert obsolete recommendations. Finally, CDSS must capture clinical complexity (ie, including dimensions such as oncogeriatrics) and patient preferences. Currently, there is no shared vision about whether CDSS should be oriented toward ‘simpler’ or ‘more complex’ cases, nor whether a CDSS can include existing information on open clinical trials.
Provision of patients’ genomics information and molecular tumour boards
The emergence of personalised medicine can impact decision-making in MTMs. The idea of implementing molecular tumour boards (comprised of specialists in genetics, biology, medical oncology, bioinformatics and pathology) has emerged due to the complexity of selecting patients and evaluating different options according to the information provided by next-generation sequencing. But integrating this area into MTMs poses specific challenges beyond the technical challenges of improving clinical decisions. For one, MTMs must access genomic information, and hospitals do not always have this technology onsite, making virtual MTMs necessary. Moreover, the interpretation of genomic information must be consistent with overall therapeutic planning, including indications for drugs.
Virtual MTMs
Virtual MTMs facilitate regular, multicentre meetings, but informants stressed that virtual MTMs do not justify delivering treatments in local centres that may not be able to guarantee adequate quality of care or patients’ access to clinical trials. However, they can serve to reach a consensus and coordinate provision of chemotherapy or patient follow-up in the local centre. Furthermore, asynchronous MTMs—discussing cases without involving the other institution in real-time—were seen as problematic; efforts to save time should be focused on making synchronous MTMs more efficient rather than using an asynchronous model.
An inherent problem of virtual MTMs is confidentiality when accessing clinical data in patients receiving treatment in other hospitals, particularly when local legislation follows the European General Data Protection Regulation. Some informants reported having to fill in a consent form in order to communicate and exchange patient information between centres, while others did not. A few pointed out that an interhospital HIS averts this obstacle. Another example of how to address this issue is to send a link that is configured to expire within hours to patients’ EHRs on referral.
Recording of decisions and outcome evaluation
MTM decisions and minutes
Decision-making in MTMs produces information and medical summons for the patient. On the information side, most team decisions are recorded in the patient’s EHR and generally reflected in the treatment strategy and in other medical decisions. This makes the information accessible in the hospital context. However, decisions are normally recorded in the same free-text format used for other data, limiting their subsequent use as information inputs that can be assessed in terms of clinical outcomes or team performance in the medium to long term. The MTM minutes or reports synthesise the team’s collective reasoning and any potential divergences among its members. They also follow a free-text format, which was seen as difficult to change considering the need to qualify decisions and acknowledge discrepancies.
Management of patient appointments
Regardless of the administrative support that MTMs have, patient summons can be facilitated by HIS that allow agile, real-time management. Ideally, appointment summons generated during the MTM should be automatically incorporated into the hospital agenda rather than being a pending action point for after the meeting. Many teams, however, cannot perform this task in situ, increasing the postmeeting workload.
Evaluation of MDT outcomes
ICTs have had a negligible impact on evaluation of MDT activities and outcomes. It is not unusual to see the generation of independent Excel files recording MDTs’ outcomes—with approval of ethical committee and informed consent of patients—unconnected from the HIS interface of other operating systems. These experiences often depend solely on personal efforts, sometimes related to publications; they are not systematised. Furthermore, the records are usually generated retrospectively, entailing added work and potential errors. Exceptionally, hospital HIS include evaluation systems that automatically measure toxicity, stages (I, II…), or other intermediate and outcome indicators. But these experiences are limited in number. As those functionalities are overwhelmingly related to the generation of structured data points, they cannot capture the context of free-text records. Paradoxically, this situation predominates in conventional patient care, while in clinical trials the activity registries are far more standardised and structured.
After analysing the data, the set of ICTs and care components studied was synthesised on the basis of the four domains in figure 2.
Figure 2.
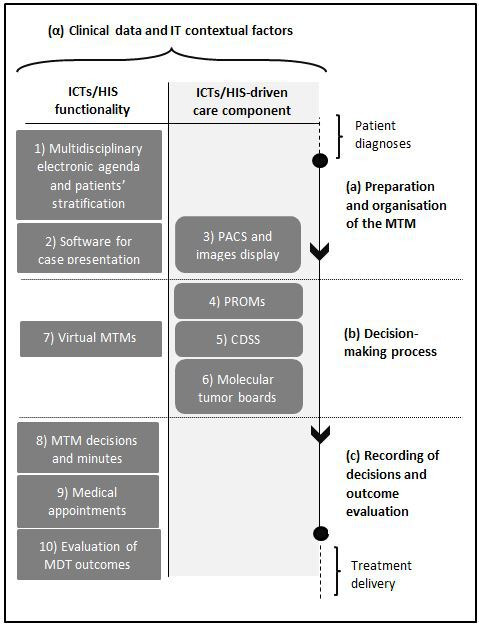
Information and communication technologies (ICTs) and care components used during the multidisciplinary team meeting (MTM) stages. The column on the right defines the three stages (a–b–c) of informational and decision-making processes related to MTMs, from preparation to outcome evaluation. The ICT/health information system (HIS) functionalities (left column) and ICTs-driven care components (central column) are shown stage by stage. The contextual factors are displayed at the top as a transversal domain. CDSS, clinical decision support systems; IT, information technology; MDT, multidisciplinary team; PACS, picture archiving and communication system; PROM, patient-reported outcome measure.
Discussion
This study found 10 ICT/HIS functionalities and ICT-driven care components that to a greater or lesser extent have been adopted and impact MTMs informational and decision-making processes. Our results indicate that ICTs play a key role in opening MTMs to other institutions and departments (by means of virtual MTMs and molecular tumour boards) as well as to patients through data registries that have an impact on these processes in real time (eg, PROMs). ICTs also contribute to increasing the internal efficiency of teams, for example, through multidisciplinary electronic agendas to draw up patient lists or through structured, personalised case presentations. These technologies are also enabling the use of operating systems intended to improve MTM decisions (eg, real-world data in CDSS) and contribute to assessing team performance. Although the degree of adoption of ICTs and care components is uneven among different European health systems and there is a high variability,17 our results showed common trends in digital, dynamic interaction between team members and the larger health ecosystem (beyond the hospital setting), and the integration of patient inputs and support systems as well as from physician-generated information. Globally, this situation paves the way to transform MTM model away from a decision-making process bound within an isolated room, and mark a second transition in the process of MDT development.
That said, our study highlights the low concordance between MDTs’ information needs and the adequacy of current IT context. Hospital HIS are still based on reports and clinical services, rather than organised along care processes, and the combination of ‘passive’ HIS and EHRs—conceived as instruments to store and classify information, not to work with it—plus the massive generation of unstructured data in the form of free-text pdf files, is the clearest expression of this gap. Keen describes this mismatch, noting that while health services are increasingly based on a network model, where health professionals and service managers coordinate multiple services on behalf of patients, many digital services are still being designed in line with a bureaucratic data processing model.18 Because ICT use may be suboptimal, other authors call for identifying how ICTs can be implemented effectively in multidisciplinary cancer care.8 19 One example of this misalignment was revealed by a European Society of Radiology survey, which showed that only 44% of the PACS in Europe are connected to a video projector enabling direct visualisation of images during the MTM.20 Significantly, video conferencing technology and case preparation are among the 10 most-cited factors influencing MTMs’ decision-making.21 In this context, private companies have taken the initiative in developing software platforms to standardise patient data collection and case presentation.
While open dialogue continues to be the cornerstone of MTMs, the form of this dialogue is more and more intertwined with the context. One of the informants recalled that ‘in the old times we were just sitting next to each other, discussing the files, looking at the images, and someone was moderating the session’ (box 1). Since the hypothesis arising from our research is that the MTM model is in transition, it is worth outlining some critical aspects of this emerging model.
First, the MTM coordinator, whose overarching role is to manage patient lists and promote clinical consensus, could also potentially assume functions related to synchronising the team and the different interfaces (molecular tumour boards, virtual MTM) along with the inputs generated or facilitated by ICTs (CDSS, PROMs). This figure could also proactively manage the patient agenda, for instance by validating the stratification of cases proposed by different professionals. This aspect is especially urgent considering the increasing incidence of malignancies and the evident management challenges involved in guaranteeing a reasonable time period to discuss clinically complex cases in a multidisciplinary forum. A Dutch study analysed 105 000 cancer cases to identify pathways for increasing health system efficiency and proposed stratifying cases in three levels according to the need for multidisciplinary evaluation.22
Second, the current proliferation of ICTs and care components in the MTM context requires rationalisation of their use based on medical criteria—not only technological feasibility. For instance, the use of artificial intelligence (or deep learning) in CDSS illustrates the ethical dilemmas and misgivings that can arise. As other authors stressed, while discussion remains active on how AI could ‘revolutionise’ healthcare delivery, there is a lack of direction and evidence on how AI could actually benefit patients.23 The use of ICTs was clearly accelerated during the COVID-19 pandemic. Recent evaluations in the UK led some authors to suggest that virtual MTMs will be an alternative to face-to-face meetings and a standard component of future clinical workflows,24 while others request caution since quality of the multidisciplinary discussion was hampered.25
Finally, the transition towards a new MTM model, more connected to its surroundings and capable of integrating different kinds of information, will lag unless HIS overcome current limitations for providing structured data, allowing MDTs to assess their performance and outcomes.
Additionally, while it is desirable for organisationally and culturally mature MDTs to integrate ICTs that increase their effectiveness and efficiency, the adoption of ICTs does not preclude professionals’ and MDTs’ need for support. These technologies may generate an additional workload for professionals, especially when they are being introduced. A data manager or administrative or IT support should accompany the implementation and use of ICTs, especially when (as observed in our study) interoperability problems between HIS from different hospitals already impose a heavy workload. Interhospital referrals and discussions are increasing, buoyed by regionalisation of services, centralisation policies and networks that share care processes among different hospitals. The relevant experience of the European reference networks for rare diseases stand out in this respect, representing a practical model through which teams from different countries share information and make decisions using an approach fully reliant on ICTs.26 27
This study has both strengths and limitations. One strength relates to the criteria used to select the sample, which included interviewees from different specialties and health systems. Moreover, to avoid social desirability bias, where participants might misrepresent their improvement efforts to provide desirable answers,28 we asked informants to describe both positive and negative experiences when presenting their cases. In the case of European Society of Medical Oncology (ESMO) and European Society of Gynaecological Oncology, the participants were selected specifically by the researchers since ESMO do not belong to ECCO and the surgical societies did not react to the initiative. Regarding the limitations, the small number of participants meant it was impossible to capture all ICT functionalities and care components being used in MTMs. Also, as the study was exploratory by nature, we did not achieve data saturation. Another potential limitation relates to the participant selection process, based on proposals put forward by each scientific society, which could have biased selection towards individuals who had had successful experiences. Finally, one scientific society did not find the adequate professional profile to be involved in the study.
The participants in the workshop became coauthors of this study, thereby giving rise to potential participant bias. Relevantly, they were proposed as coauthors once the workshop was held, so data collection was not altered. In general, this shift in their position implied two adjustments: first, the preliminary results—including the process of thematic analysis—were disclosed to them but, in order to avoid the research bias, they were allowed to discuss their interpretation only in the Discussion (ie, their views did not affect the results and the selected verbatim), which is a limitation. Hence, they were offered to resign as coauthors, if disagree. Second, it should be noted that the researchers leading the study openly discussed the implications of the results as well as the conclusions of the study on an equal basis with the invited coauthors.
In brief, ICTs and associated care components are transforming informational and decision-making processes along the three stages of MTM development. Factors driving their introduction include the increased personalisation required by clinical and care approaches as well as the need for more efficiency in MTM informational processes. The emerging MTM model is better integrated in the wider health system context (beyond the hospital setting) and better equipped to incorporate inputs from patients and support systems, making MTMs more dynamic and interconnected. While these changes signal a second transition in the development process of MDTs, they are occurring in a context marked by gaps between MDTs’ information and management needs and the adequacy of current IT systems. This situation needs to change before MDTs can develop their full potential.
Supplementary Material
Acknowledgments
We should like to thank Ricard Price who so unstintingly shared his thoughts with us and supported the organisation of the study field. Further, we are grateful to Ms. Meggan Harris for her editorial support.
Footnotes
Contributors: JP and JMB conceptualised this study. LDL, KG, EJ, CL, JdM, JP, DR, RS, VV, CC-O and JP made substantial contributions to the acquisition and analysis of data for the work. JP and CC-O wrote the draft, and JMB supervised the manuscript. JP, CC-O, LDL, KG, EJ, CL, JdM, JP, DR, RS, VV and JMB provided intellectual content, edited the manuscript, approved the final version for submission and agree to be accountable for all aspects of the work. JP, CC-O and JMB managed the overall design of the study and also had primary responsibility for the final content.
Funding: This work was supported by the Innovative Partnership for Action Against Cancer Joint Action (Grant Agreement number: 801520—iPAAC—HP-JA-2017), which has received funding from the European Union through the Consumers, Health, Agriculture and Food Executive Agency of the European Commission, in the framework of the Health Programme 2014-2020. This work was also supported by the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR, 2017SGR735), Government of Catalonia, Spain.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Qualitative data generated in this study will not be reused under any conditions.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants but The study was strictly based on experts’ opinion. Recorded information cannot be linked to a subject. The study is exploratory in nature and it contains no information on specific patients, clinical practice issues or management situations. The disclosure of responses outside of the research cannot place any subject at risk. All the participants signed the informed consent. Confidentiality was ensured by the removal of names and other identifying information from transcriptions and analyses. Participants gave informed consent to participate in the study before taking part.
References
- 1.European Partnership Action Against Cancer consensus group, Borras JM, Albreht T, et al. Policy statement on multidisciplinary cancer care. Eur J Cancer 2014;50:475–80. 10.1016/j.ejca.2013.11.012 [DOI] [PubMed] [Google Scholar]
- 2.Saini KS, Taylor C, Ramirez A-J, et al. Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries. Ann Oncol 2012;23:853–9. 10.1093/annonc/mdr352 [DOI] [PubMed] [Google Scholar]
- 3.Prades J, Remue E, van Hoof E, et al. Is it worth reorganising cancer services on the basis of multidisciplinary teams (MDTs)? A systematic review of the objectives and organisation of MDTs and their impact on patient outcomes. Health Policy 2015;119:464–74. 10.1016/j.healthpol.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 4.Berghmans T, Lievens Y, Aapro M, et al. European cancer organisation essential requirements for quality cancer care (ERQCC): lung cancer. Lung Cancer 2020;150:221–39. 10.1016/j.lungcan.2020.08.017 [DOI] [PubMed] [Google Scholar]
- 5.Andritsch E, Beishon M, Bielack S, et al. ECCO essential requirements for quality cancer care: soft tissue sarcoma in adults and bone sarcoma. A critical review. Crit Rev Oncol Hematol 2017;110:94–105. 10.1016/j.critrevonc.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 6.Wilson ARM, Marotti L, Bianchi S, et al. The requirements of a specialist breast centre. Eur J Cancer 2013;49:3579–87. 10.1016/j.ejca.2013.07.017 [DOI] [PubMed] [Google Scholar]
- 7.Organisation of European Cancer Institutes (OECI) . Standards (accreditation and designation programme), 2019. Available: https://www.oeci.eu/accreditation/Page.aspx?name=OECI_STANDARDS
- 8.Janssen A, Brunner M, Keep M, et al. Interdisciplinary eHealth practice in cancer care: a review of the literature. Int J Environ Res Public Health 2017;14:1289. 10.3390/ijerph14111289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kutikov A, Weinberg DS, Edelman MJ, et al. A war on two fronts: cancer care in the time of COVID-19. Ann Intern Med 2020;172:756–8. 10.7326/M20-1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tepper DL, Burger AP, Weissman MA. Hands down, COVID-19 will change medical practice. Am J Manag Care 2020;26:e274–5. 10.37765/ajmc.2020.88478 [DOI] [PubMed] [Google Scholar]
- 11.Kitzinger J. Qualitative research. Introducing focus groups. BMJ 1995;311:299–302. 10.1136/bmj.311.7000.299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlsen B, Glenton C. What about N? A methodological study of sample-size reporting in focus group studies. BMC Med Res Methodol 2011;11:26. 10.1186/1471-2288-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sofaer S. Qualitative research methods. Int J Qual Health Care 2002;14:329–36. 10.1093/intqhc/14.4.329 [DOI] [PubMed] [Google Scholar]
- 14.Miles M, Huberman A. Qualitative data analysis. Newbury Park, CA: Sage Publications, 1994. [Google Scholar]
- 15.Muhr T. ATLAS.ti 9 for windows. Berlin: Scientific Software Development, 2011. [Google Scholar]
- 16.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care 2007;19:349–57. 10.1093/intqhc/mzm042 [DOI] [PubMed] [Google Scholar]
- 17.World Health Organisation (WHO) . From innovation to implementation. eHealth in the European region. Copenhagen Denmark: WHO Regional Office for Europe, 2016. http://www.euro.who.int/en/ehealth [Google Scholar]
- 18.Keen J. Digital health care: cementing centralisation? Health Informatics J 2014;20:168–75. 10.1177/1460458213494033 [DOI] [PubMed] [Google Scholar]
- 19.Janssen A, Robinson T, Brunner M, et al. Multidisciplinary teams and ICT: a qualitative study exploring the use of technology and its impact on multidisciplinary team meetings. BMC Health Serv Res 2018;18:444. 10.1186/s12913-018-3242-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neri E, Gabelloni M, Bäuerle T, et al. Involvement of radiologists in oncologic multidisciplinary team meetings: an international survey by the European Society of oncologic imaging. Eur Radiol 2021;31:983–91. 10.1007/s00330-020-07178-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soukup T, Lamb BW, Arora S, et al. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc 2018;11:49–61. 10.2147/JMDH.S117945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walraven JEW, Desar IME, Hoeven van der JJM, et al. Analysis of 105.000 patients with cancer: have they been discussed in oncologic multidisciplinary team meetings? A nationwide population-based study in the Netherlands. Eur J Cancer 2019;121:85–93. 10.1016/j.ejca.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 23.Lau AYS, Staccini P, Section Editors for the IMIA Yearbook Section on Education and Consumer Health Informatics . Artificial intelligence in health: new opportunities, challenges, and practical implications. Yearb Med Inform 2019;28:174–8. 10.1055/s-0039-1677935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sidpra J, Chhabda S, Gaier C, et al. Virtual multidisciplinary team meetings in the age of COVID-19: an effective and pragmatic alternative. Quant Imaging Med Surg 2020;10:1204–7. 10.21037/qims-20-638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.To N, Bekker HL, Henry K, et al. COVID-19 restrictions on multidisciplinary team meeting decision-making: service evaluation in a major UK cancer centre. Br J Surg 2021;108:e162–3. 10.1093/bjs/znab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.European Commission, . European reference networks (Erns), 2018. Available: https://ec.europa.eu/health/ern_en
- 27.Joint Action on Rare Cancer (JARC) . Rare Cancer Agenda 2030 - Ten Recommendations from the EU Joint Action on Rare Cancers, 2018. Available: https://www.jointactionrarecancers.eu/attachments/article/265/Rare_Cancer_Agenda_2030.pdf
- 28.Sudman S, Bradburn NM, Schwartz N. Thinking about answers: the application of cognitive processes to survey methodology. San Francisco: Jossey-Bass, 1996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information. Qualitative data generated in this study will not be reused under any conditions.


