FIGURE 2.
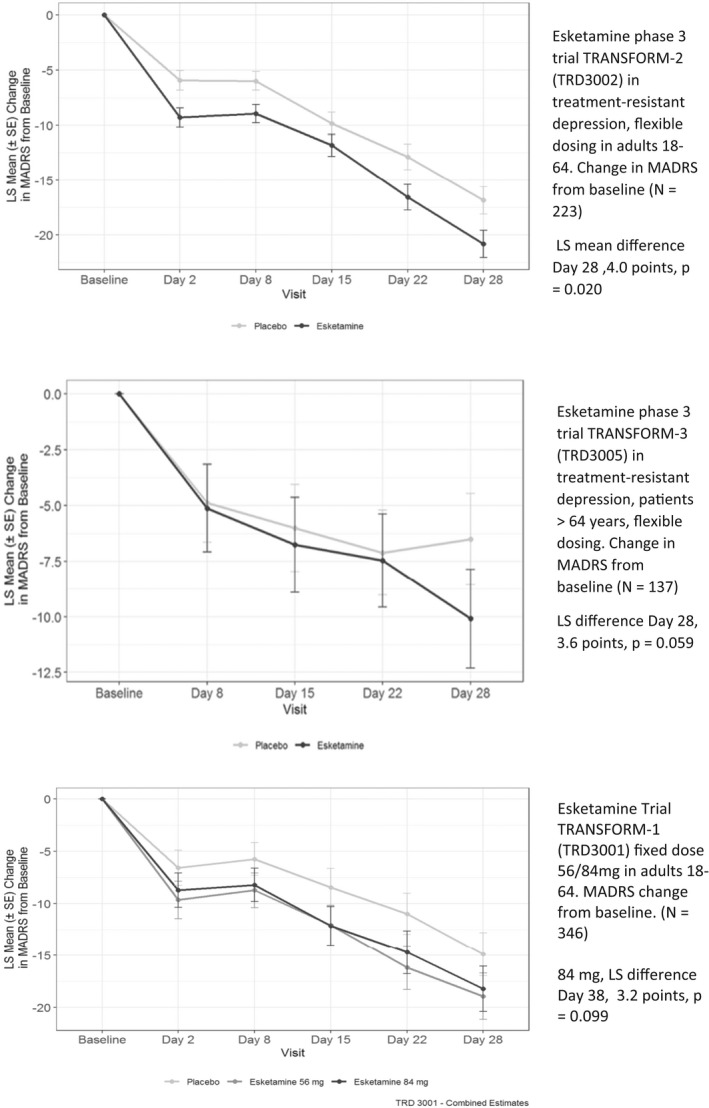
Successful and unsuccessful phase 3 clinical trials of esketamine for treatment‐resistant depression. Note: Both treatment and placebo patients switched to a new antidepressant at baseline. Abbreviations: MADRS, Montgomery‐Asberg Depression Rating Scale; LS Means, least squares means; SE, standard error. Flexible Dosing = 56‐84 mg. Source: Adapted from Kim J, Chen Q27
