FIGURE 3.
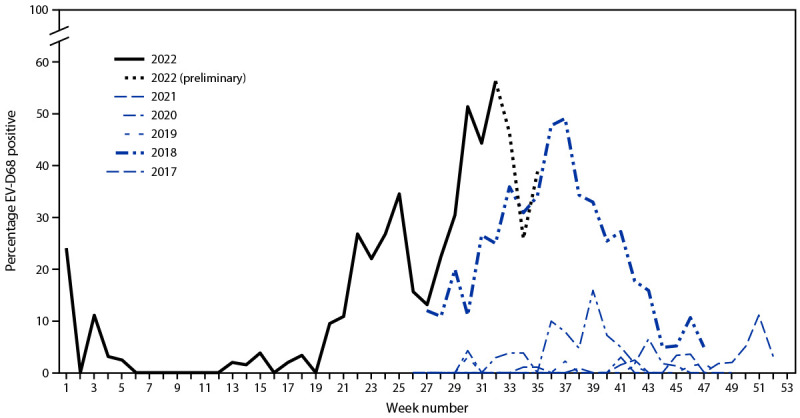
Weekly trends in reported percentage of positive enterovirus D68 test results among children and adolescents aged <18 years with acute respiratory illness and positive rhinovirus/enterovirus test results who received care in the emergency department or inpatient units — New Vaccine Surveillance Network,* United States, 2017–2022†
Abbreviation: EV-D68 = enterovirus D68.
* The seven sites in the New Vaccine Surveillance Network are located in Kansas City, Missouri; Rochester, New York; Cincinnati, Ohio; Pittsburgh, Pennsylvania; Nashville, Tennessee; Houston, Texas; and Seattle, Washington. Two sites do parallel testing with a pan-rhinovirus and EV-D68 assay; fives sites do sequential testing with a pan-rhinovirus and pan-enterovirus assay or a rhinovirus/enterovirus assay, followed by an EV-D68 assay. All sites use the same CDC-developed EV-D68 reverse-transcription–polymerase chain reaction assay.
† Testing for EV-D68 occurred at all seven sites during July–October 2017 and during July–November 2018–2020. Year-round testing began at most sites in July 2021 and was fully implemented at all sites during June 2022. EV-D68 testing windows in NVSN have changed over time, limiting annual comparisons outside of these windows. Retrospective testing is still in process for 2021 and early 2022, and data are current as of September 22, 2022. Weeks 33–35 are subject to delays in reporting.
