Abstract
Background
Prurigo nodularis is a debilitating skin condition that is classified as rare by the Genetic and Rare Diseases Information Center (GARD) and the National Organization for Rare Diseases (NORD). There are currently no estimates of the prevalence of prurigo nodularis in England.
Objectives
We aimed to address this data gap by describing the epidemiology of prurigo nodularis in a representative dataset derived from the English National Health Service.
Methods
The study utilized data from the Clinical Practice Research Datalink linked to Hospital Episode Statistics inpatient data. Patients with a diagnosis of prurigo nodularis were selected by clinical code in the primary care or inpatient datasets. Case definition was based on a minimum of two distinct diagnoses to maximize specificity. Point prevalence was calculated for the midpoint of 2018 and incidence rates from 2008 to 2018 were presented. For those classified as incident cases, demographic and clinical characteristics were reported. In sensitivity analyses the case definition was modified to relax the multiple diagnosis criteria and to restrict cases to those diagnosed within a maximum of 4 or 10 years of the midpoint prevalence date.
Results
Overall, 11 656 patients within the dataset had at least one prurigo nodularis diagnosis. Following application of the relevant inclusion criteria, 2743 patients formed the point prevalent cohort; the estimated prevalence was 3·27 patients per 10 000 population [95% confidence interval (CI) 3·15–3·40]. In sensitivity analyses the estimated prevalence ranged from 2·24 (95% CI 2·14–2·34) to 6·98 (95% CI 6·80–7·16). Incidence over the study period was 2·88 per 100 000 patient‐years. Comorbidity was relatively high in this population, notably for atopic dermatitis (52·2%), depression (41·1%) and anxiety (35·4%).
Conclusions
This study supports the NORD/GARD classification of prurigo nodularis as a rare disease with a prevalence of 3·27 patients per 10 000 population, which equates to 18 471 patients living with the disease in England in 2018. The relatively high prevalence of comorbidity observed for these patients may increase the complexity of management.

Prurigo nodularis, alternatively known as nodular prurigo, is a chronic disease characterized by multiple hyperkeratotic nodules and papules. 1 These lesions are severely pruritic, and are typically symmetrically distributed along a patient’s extremities. 2 , 3 At present, the mechanisms underlying prurigo nodularis remain poorly understood. Continuous itching itself is believed to trigger prurigo nodularis, and this behaviour is often routed to dermatological, systemic, infectious or psychiatric disorders. However, in many cases, no underlying condition is identified. Prurigo nodularis has a major impact on patient quality of life 4 and appears to affect a disproportionate number of female patients. Presentation at clinic has been reported to occur most often between the ages of 51 and 65 years. 5
Prurigo nodularis is classified as a rare disease by the National Institutes of Health Genetic and Rare Diseases Information Center (GARD) 6 and National Organization for Rare Diseases (NORD). 7 In the UK, the prevalence of prurigo nodularis is unknown as there are no studies describing the epidemiology of the condition. In the USA, prevalence estimates range from 3·6 to 14·8 per 10 000 population, 8 , 9 whereas in the European context, rates of between 0·65 and 11·1 per 10 000 population have been reported. 10 , 11 , 12
In addition to natural variation, this relatively wide range of estimates is partly due to differences in case definition and the representativeness of the study populations. The International Classification of Diseases (ICD), Ninth Revision, Clinical Modification (ICD‐9‐CM), 13 used extensively in the USA until 2015, did not have a specific diagnostic code for prurigo nodularis. Therefore, studies using this classification based their definition on the ICD‐9‐CM code 698·3 (lichenification and lichen simplex chronicus), 9 , 14 and consequently overestimated the prevalence, as other conditions that appear under this umbrella term would have been included. One study 14 attempted to assign the proportion of prurigo nodularis cases that are likely to be captured by this code, but such methods are somewhat arbitrary and subject to bias of clinical setting. In addition, previous prevalence estimates have been derived from either distinct age‐defined subsets of the population, 8 , 9 specific healthcare settings 10 , 11 or from data sources derived from insurance‐based sources, 8 , 9 , 12 which have inherent demographic biases and are therefore not representative of the underlying population.
To address this data gap, we aimed to estimate the incidence and prevalence of prurigo nodularis in England using the Clinical Practice Research Datalink (CPRD) (Aurum) dataset. CPRD Aurum is derived from the UK National Health Service (NHS), which treats the vast majority of the UK population, especially primary care contacts, and therefore avoids the demographic selection biases of other studies. In addition, data within CPRD Aurum, which contains both primary and secondary care data, uses the Systematized Nomenclature of Medicine Clinical Terms (SNOMED‐CT) 15 and ICD 10th Revision (ICD‐10), 16 both of which have specific diagnostic codes for prurigo nodularis.
Patients and methods
Data source
The study was a retrospective, descriptive database analysis conducted using the CPRD Aurum database, 17 a longitudinal, anonymized research database containing data on approximately 40 million patients derived from primary care practices in the UK. The Aurum primary care dataset comprises data including demographics, diagnoses, hospital referrals and prescriptions originating from primary care. For practices in England, data are linked to additional sources including the Hospital Episode Statistics (HES) 18 admitted patient care (APC) dataset, which contains data for all inpatient admissions occurring within NHS hospitals in England, and the Office for National Statistics (ONS) mortality dataset, 19 which contains death registration data for all deaths in England. There is a time lag between when the primary care practice submits data to the CPRD and when the patient’s data are linked, therefore not all English patients are currently linked. In addition, data for some patients cannot be linked owing to missing data items required for the linkage process.
Diagnostic information in the CPRD Aurum primary care dataset is recorded using medcodes that map to the Read 20 and SNOMED‐CT classifications. 15 HES inpatient data are recorded using the ICD‐10 classification. 16 Data quality is ascribed in CPRD using an acceptable status flag. Patient data are classified as acceptable for research if they have a valid coding for sex, have a valid birth year with no prior activity recorded before this date, and an age of <115 years at last data collection point. Patients must also be permanently registered at the practice.
Studies using CPRD are covered by ethics approval, granted by the Trent Multicentre Research Ethics Committee (Reference 05/MRE04/87). CPRD Independent Scientific Advisory Committee approval was granted for this study (ISAC 20‐166).
Sample size considerations
For the primary outcome of estimated prevalence of prurigo nodularis, based on 0·01% precision and 95% confidence and an estimated prevalence of 0·07%, the required denominator would be 268 715.
Study population
Patients with prurigo nodularis were selected from both the CPRD Aurum and HES‐linked APC datasets. Exposure was defined as the presence of a diagnosis of prurigo nodularis as ascertained by medcode (198021000006113/3532811000006115) or ICD‐10 code (L28·1) in the Aurum or HES APC datasets, respectively. To avoid false‐positives resulting from the inclusion of patients with diagnoses recorded for exploratory investigation prior to specialist confirmation, an additional diagnosis of prurigo nodularis was required to be recorded on a subsequent date. Patients also had to meet the following criteria:
Acceptable patient record as defined by CPRD in the patient table to ensure only patient records of acceptable research quality were used in the study in both the numerator and the denominators.
Eligible for linkage to the HES and ONS datasets to capture inpatient diagnoses of prurigo nodularis from the HES dataset and to capture date of death from the ONS dataset accurately.
For the cross‐sectional prevalence analysis, patients currently registered at an Aurum practice on 30 June 2018 (midpoint date) and with a first diagnosis of prurigo nodularis prior to or on this date, formed the study cohort. As prurigo nodularis is a chronic disease without a licensed treatment or natural resolution, there were no limitations on prior duration between last prurigo nodularis diagnosis and the midpoint date, but this was explored further in the sensitivity analyses.
Sensitivity analyses
Owing to the accepted limitations of routine data, a series of sensitivity analyses were conducted based on combinations of the following criteria:
Patients were only required to have one diagnosis of prurigo nodularis in either the Aurum primary care or HES APC dataset as classified above.
Patients were required to have a diagnosis of prurigo nodularis recorded within either: (i) 4 years or (ii) 10 years prior to the midpoint date. These timeframes attempted to capture two reasonable scenarios for which a patient with prurigo nodularis would consult primary care for their condition or would have secondary care data fed back into their patient record.
For the incident population, patients with a first diagnosis of prurigo nodularis between 2008 and 2018 were extracted. Patients were required to be registered at the primary care practice for a minimum of 90 days prior to first diagnosis in order to be included within the incident cohort. Previous exploration of CPRD data has demonstrated that a period of ~90 days is sufficient from the time of the patient’s registration for the patient to be reviewed by their new primary care practice and have all pre‐existing significant morbidities recorded. Thus, it was assumed that new diagnoses recorded after 90 days were incident diagnoses. The date of first prurigo nodularis diagnosis defined the incidence index date.
Analysis
Point prevalence of prurigo nodularis was calculated for 2018. Cases with the first diagnosis of prurigo nodularis occurring prior to 30 June 2018 and with an active registration with a CPRD Aurum practice on this date formed the numerator population. The denominator was all patients registered with a CPRD Aurum practice on this date matching the same criteria as the cases, i.e. acceptable patient status and being eligible for the HES linkage scheme.
To estimate the number of prurigo nodularis cases in England, observed 2018 prevalence rates from this analysis were stratified by age (in 10‐year age bands) and sex and were applied to the ONS England population estimate for 2018. 21 This allowed us to extrapolate the number of cases in each age and sex category. These subtotals were aggregated to estimate the total number of age‐ and sex‐adjusted point prevalent prurigo nodularis cases in England in 2018.
Sensitivity analyses were conducted by changing the case definition as described above and replicated in a matrix to create three additional scenarios.
Incidence of prurigo nodularis was calculated from 2008 to 2018, using new cases diagnosed per year as the numerator with the aggregated person‐time by year observed in CPRD for patients of acceptable status as the denominator. This was calculated as the difference between either 1 January 2008 or the patient’s registration date in each year, whichever was latest, and 31 December 2018 or the patient’s last follow‐up date (defined as earliest of either the date the patient left their primary care practice or the last data collection date for their practice).
For those patients in the incident cohort, demographic and clinical data at date of first recorded prurigo nodularis diagnosis were presented. Comorbidities (atopic dermatitis, depression, anxiety, HIV infection, hepatitis, congestive heart failure, chronic kidney disease, type 2 diabetes mellitus) recorded prior to index date were ascertained by SNOMED‐CT or ICD‐10 code in the Aurum or HES APC datasets, respectively.
Results
Prevalence
The June 2020 build of CPRD Aurum contained records for 42 108 395 patients. Of these, 11 656 had a relevant diagnosis for prurigo nodularis ever recorded in the dataset. Following application of the relevant inclusion criteria and requirement for patients to be registered and diagnosed prior to 30 June 2018 (inclusive) there were 2743 patients in the point prevalent cohort (Table 1). Of those patients in the point prevalent cohort, 2668 (97·3%) had diagnoses recorded in the Aurum primary care dataset and 453 (16·5%) had diagnoses recorded in the HES APC inpatient dataset.
Table 1.
Derivation of the 2018 point prevalent population with prurigo nodularis from the Clinical Practice Research Datalink (CPRD) Aurum database
| Patients included, n (%)a | Patients excluded, n (%)a | |
|---|---|---|
| All patients registered in CPRD | 42 108 395 | – |
| Acceptable CPRD status | 34 266 984 (81·4) | 7 841 411 (18·6) |
| Patient registered at primary care practice in England | 34 169 199 (99·7) | 97 785 (0·3) |
| Patient eligible for HES/ONS linkage | 21 264 007 (62·2) | 12 905 192 (37·8) |
| Recorded diagnosis of prurigo nodularisb | 11 656 (0·1) | 21 252 351 (99·9) |
| Diagnoses recorded prior to/on 30 June 2018 | 10 076 (86·4) | 1580 (13·6) |
| Patient currently registered on 30 June 2018 | 5852 (58·1) | 4224 (41·9) |
| Patient has subsequent, confirmatory diagnosis | 2743 (46·9) | 3109 (53·1) |
HES, Hospital Episode Statistics; ONS, Office for National Statistics. aPercentages refer to patients included/excluded from the preceding step. bDiagnosis of prurigo nodularis in either the Aurum or HES datasets.
Based on these patients, the estimated point prevalence of prurigo nodularis was 3·27 (3·15–3·40) per 10 000 population. Prevalence of prurigo nodularis was lower for male sex compared with female sex (2·54 vs. 4·01 per 10 000), and increased by age ranging from 0·19 and 0·10 per 10 000 for male sex and female sex in the youngest age group (0–14 years) to 11·57 and 13·76 in the oldest age group (≥ 85 years), respectively (Figure 1). When the age and specific prevalence rates were extrapolated to the English population, we estimated a total of 18 471 patients with prurigo nodularis (Table 2).
Fig 1.
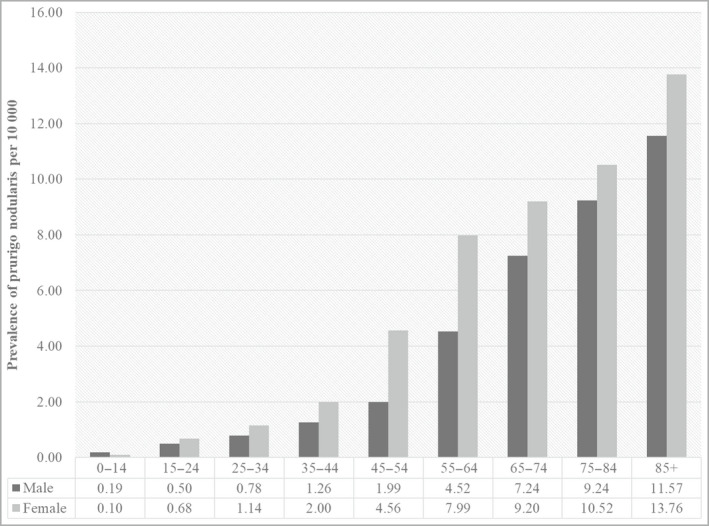
Estimated prevalence of prurigo nodularis per 10 000 population in the Clinical Practice Research Datalink Aurum Database 2018.
Age ranges are provided in years.
Table 2.
Estimated cases of prurigo nodularis in England 2018 extrapolated from the Clinical Practice Research Datalink (CPRD) Aurum database
| Age, years | CPRD derived prevalence per 10 000a | ONS 2018 midpoint population estimates 21 | Extrapolated prurigo nodularis cases for England | ||||
|---|---|---|---|---|---|---|---|
| Male sex | Female sex | Male sex | Female sex | Male sex | Female sex | Total | |
| 0–14 | 0·19 | 0·10 | 5 225 139 | 4 966 950 | 101 | 50 | 152 |
| 15–24 | 0·50 | 0·68 | 3 380 438 | 3 197 657 | 170 | 219 | 389 |
| 25–34 | 0·78 | 1·14 | 3 833 674 | 3 775 689 | 301 | 432 | 733 |
| 35–44 | 1·26 | 2·00 | 3 549 307 | 3 598 632 | 448 | 718 | 1167 |
| 45–54 | 1·99 | 4·56 | 3 766 221 | 3 857 052 | 751 | 1760 | 2510 |
| 55–64 | 4·52 | 7·99 | 3 336 851 | 3 445 635 | 1509 | 2752 | 4260 |
| 65–74 | 7·24 | 9·20 | 2 682 950 | 2 893 116 | 1943 | 2662 | 4604 |
| 75–84 | 9·24 | 10·52 | 1 536 167 | 1 844 432 | 1420 | 1940 | 3360 |
| ≥ 85 | 11·57 | 13·76 | 517 084 | 879 967 | 598 | 1211 | 1809 |
| Total | 0·19 | 0·10 | 27 827 831 | 28 459 130 | 7058 | 11 413 | 18 471 |
ONS, Office for National Statistics. aDiagnoses selected from the CPRD Aurum dataset linked to Hospital Episode Statistics admitted patient care.
Based on a series of sensitivity analyses the estimated prevalence of prurigo nodularis ranged from 2·24 (95% CI 2·14–2·34) for those with the additional requirement of having a diagnosis within 4 years of the midpoint date, to 6·98 (95% CI 6·80–7·16) for those cases requiring only a single recorded diagnosis in their patient record without any further criteria (Figure 2).
Fig 2.
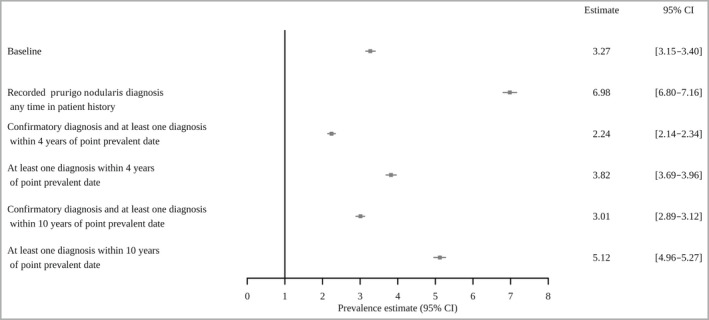
Sensitivity analysis of the estimated prevalence of prurigo nodularis per 10 000 population in the Clinical Practice Research Datalink Aurum Database 2018.
CI, confidence interval.
Incidence
Incidence of first recorded diagnosis over the study period was 2·88 (95% CI 2·77–3·00) per 100 000 patient‐years based on our base case definition. Characteristics of patients at time of incident diagnosis of prurigo nodularis between 2008 and 2018 are shown in Table 3. The mean age at first recorded diagnosis was 61·1 years (SD 18·4) and the majority of patients (58·9%) were female. There were relatively high levels of baseline comorbidity for this population, with the most common being atopic dermatitis [recorded in the patient history of over half of the patients (52·2%)] and psychiatric diagnoses [depression (41·1%) and anxiety (35·4%)]. Type 2 diabetes was a recorded diagnosis for 19·8% of patients, 17·6% of patients had a recorded diagnosis of chronic kidney disease and 21·3% had a diagnosis of coronary heart disease. Only a small proportion of patients (0·2%) had a recorded diagnosis of HIV.
Table 3.
Baseline characteristics of patients with prurigo nodularis at index diagnosis (2008–2018) with comparative data from all patients registered in the Clinical Practice Research Datalink (CPRD) in 2018
| Characteristic | Prurigo nodularis | All patients registered in CPRD in 2018 |
|---|---|---|
| N = 2416 | N = 8 378 777 | |
| Mean age, years (SD) | 61·1 (18·4) | 40·1 (23·2) |
| Sex | ||
| Male | 982 (40·3) | 4 186 951 (50·0) |
| Female | 1434 (58·9) | 4 191 662 (50·0) |
| Prior recorded diagnoses | ||
| Atopic dermatitis | 1270 (52·2) | |
| Depression | 1001 (41·1) | |
| Anxiety | 861 (35·4) | |
| HIV | 6 (0·2) | |
| Coronary heart disease | 516 (21·2) | |
| Chronic kidney disease | 428 (17·6) | |
| Type 2 diabetes | 481 (19·8) | |
| Region | ||
| East Midlands | 40 (1·6) | 235 825 (2·8) |
| East of England | 122 (5·0) | 467 611 (5·6) |
| London | 331 (13·6) | 1 427 440 (17·0) |
| North East | 99 (4·1) | 358 302 (4·3) |
| North West | 544 (22·3) | 1 362 316 (16·3) |
| South Central | 212 (8·7) | 1 012 406 (12·1) |
| South East Coast | 142 (5·8) | 629 149 (7·5) |
| South West | 310 (12·7) | 1 095 822 (13·1) |
| West Midlands | 505 (20·7) | 1 450 855 (17·3) |
| Yorkshire and the Humber | 111 (4·6) | 335 480 (4·0) |
| Ethnicity | ||
| Bangladeshi | 26 (1·1) | |
| Black African | 50 (2·1) | |
| Black Caribbean | 45 (1·9) | |
| Black other | 20 (0·8) | |
| Chinese | 12 (0·5) | |
| Indian | 81 (3·4) | |
| Mixed | 28 (1·2) | |
| Other Asian | 39 (1·6) | |
| Other | 18 (0·7) | |
| Pakistani | 65 (2·7) | |
| Unknown | 10 (0·4) | |
| White | 2000 (82·8) | |
| Missing data | 22 (0·9) | |
Data are presented as n (%) unless otherwise stated.
Discussion
This retrospective database study is the first study to report the prevalence and incidence of prurigo nodularis in England. The estimated prevalence of prurigo nodularis, based on our base definition, was 3·27 (95% CI 3·15–3·40) per 10 000 patients and the estimated incidence was 2·88 (95% CI 2·77–3·00) per 100 000 patient‐years. By applying our age‐ and sex‐specific prevalence rates to the ONS English population estimate, we extrapolated a total of 18 471 patients were living with prurigo nodularis in England in 2018.
Our base case definition required patients to have two distinct diagnoses of prurigo nodularis recorded in their patient history. This was to ensure that patients who were, for example, being referred from primary care to secondary care for further investigation of a provisional diagnosis were not being incorrectly included within the case population. It has been reported that a majority of patients with prurigo nodularis (68%) have between two and four specialist referrals per year 22 and we consider it unlikely that, for a condition with such comparatively frequent healthcare contacts, cases would have only one recorded diagnosis in their patient record. We do accept that some true positive cases may have been excluded. In addition, this criterion would have led to a degree of immortality bias, in that patients were required to remain registered within the CPRD Aurum database long enough for these two diagnoses to be recorded. To test our base definition we ran a series of sensitivity analyses that: (i) relaxed the criteria for multiple diagnoses and (ii) restricted the inclusion criteria to those cases with a diagnosis within either 4 years or 10 years of the midpoint prevalence date. This created a range of estimates from 2·24 to 6·98 per 10 000 population.
It is difficult to compare our estimates with estimates from other studies owing to differences in case definition and denominator populations. A German study reported a prevalence of prurigo nodularis of 11·1 per 10 000 people; 12 however, this was based on only one required recorded diagnosis of prurigo nodularis and may thus have included exploratory cases, which would inflate the prevalence estimate. Two studies with cases selected from hospital settings have reported considerably lower prevalence figures. A Polish study reported figures of 0·65 and 0·93 per 10 000 depending on the granularity of the coding used in the case definition. 10 Similarly, a study based in Britany, France reported a prevalence of 0·8 per 10 000. 11 While it is likely that patients with prurigo nodularis will be seen in a secondary care setting, both of these studies had only relatively short study durations and thus may have omitted existing, older and less severe cases that were largely managed within primary care.
In the USA, 8 a study restricted to those aged 18–64 years who had a diagnosis specifically for prurigo nodularis, prevalence was estimated at 7·2 per 10 000, but this study only selected a distinct demographic from an insurance database, which may not be generalizable to the population as a whole. Another US study by Ständer et al. 9 considered several different data sources using both ICD‐9‐CM and ICD‐10‐CM coding systems to estimate a prevalence range between 3·7 and 4·4 per 10 000 in broadly representative populations, and 14·8 per 10 000 in a nonrepresentative elderly population. Of these estimates, the most representative was that of 4·4 per 10 000, as this was based on the Symphony Health database, which has coverage of approximately 85% of the US population and is broadly similar to our estimate of 3·27 per 10 000.
This study confirms the classification of prurigo nodularis as a rare condition attributed by NORD 6 and GARD. 7 In the USA, conditions are considered rare if they impact 200 000 people at any given time. From the study by Ständer et al., 9 the estimated prevalence of 4·4 per 10 000 would equate to 144 000 cases of prurigo nodularis in the USA based on an estimated population of 330 000 000; our estimate would equate to 108 000 cases, while our range from the sensitivity analysis would be between 73 800 and 230 400. Differences in age, sex and ethnic characteristics between the English and US populations should be considered in making this crude extrapolation.
The demographic profile of our population at incident diagnosis was broadly similar to that of other studies of prurigo nodularis, which have also reported a mean age of ~60 years and a female : male ratio of approximately 60 : 40. 5 , 23 We also report relatively high rates of comorbidity for this population as did other previous studies. The prevalence of psychiatric comorbidity has been reported in other studies. 24 We observed that over one‐third of patients had a history of depression, and a similar proportion had a history of anxiety. A retrospective analysis based on a US claims database reported increased prevalence of mood disorders [odds ratio (OR) 2·24, 95% CI 2·05–2·46] and anxiety (OR 1·93, 95% CI 1·78–2·09) compared with matched controls. Increased rates of hospitalization and increased length of stay for psychiatric conditions have also been reported for patients with prurigo nodularis. 25 Furthermore, the increased rate of depression appears to be higher than that observed for other comorbid dermatological conditions. 12 However, it is not specified whether these are independent comorbidities or whether they are related to prurigo nodularis as an underlying factor. Thus, the data have to be interpreted with caution.
The use of CPRD, with its large, representative coverage from a unified healthcare system such as the NHS and specific coding system, avoids many of the issues associated with the prevalence studies described above. However, as with all retrospective database analyses, there are limitations that should be considered when interpreting results from this study. While CPRD encompasses both primary and secondary healthcare sources, there are certain data gaps. Diagnosis data are available in the primary care and inpatient datasets but are not available in the outpatient dataset where patients with prurigo nodularis may be referred. However, for the large majority of these patients, it is expected that diagnoses would filter into the primary care system via clinic letters and enter the electronic record.
This study estimates, for the first time, the epidemiology of prurigo nodularis in England. The reported prevalence of 3·27 per 10 000 population confirms prurigo nodularis as a rare condition, as designated by GARD and NORD. The high prevalence of comorbidity at index diagnosis of prurigo nodularis may increase the complexity of management for these patients.
Author contributions
Christopher Ll. Morgan: Conceptualization (equal); data curation (equal); formal analysis (lead); investigation (lead); methodology (lead); project administration (equal); visualization (equal); writing – original draft (lead); writing – review and editing (equal). Melissa Thomas: Methodology (supporting); writing – original draft (supporting); writing – review and editing (supporting). Sonja Ständer: Visualization (equal); writing – review and editing (equal). Zarif Jabbar‐Lopez: Conceptualization (equal); writing – review and editing (equal). Sylvie Gabriel: Conceptualization (equal); writing – review and editing (equal). Christophe Piketty: Conceptualization (equal); writing – review and editing (supporting). craig currie: Conceptualization (equal); writing – review and editing (equal). Jorge Puelles: Conceptualization (equal); methodology (equal); writing – original draft (supporting); writing – review and editing (lead).
Funding sources This study was funded by Galderma SA. Employees of Galderma SA collaborated on the study design and drafting of the manuscript.
Conflicts of interest C.M., M.T. and C.C. are employees of Pharmatelligence, a company that provides research services to a range of organizations, including the pharmaceutical industry. Pharmatelligence received funding from Galderma SA to conduct this study. Z.K.J.‐L., C.P., S.G. and J.P. are employees of Galderma SA, the company that funded this study S.S. has received research grants from Almirall, Beiersdorf, German Research Foundation (DFG), European Academy of Dermatology and Venereology, German Federal Ministry of Education and Research (BMBF), the Interdisciplinary Center for Clinical Research Münster (IZKF), Leo Pharma, Menlo, Novartis, Sanofi and Trevi. She has also been an investigator for Celldex, Clexio, Dermasence, Galderma, GSK, Kiniksa, Menlo, Trevi, Novartis and Sanofi and has provided consultancy for AbbVie, Almirall, Beiersdorf, Bellus Health, Benevolent, Bionorica, Cara, Celgene, CelloHealth, Clexio, DS Biopharma, Eli Lilly, Escient, Galderma, Grünenthal, Kiniksa, Klinge Pharma, Menlo, Sanofi, Sienna, Trevi, P.G. Unna Academy, Perrigo, Pfizer, Vanda, Vifor and WebMD.
Data availability The data that support the findings of this study are available from the Clinical Practice Research Datalink. Restrictions apply to the availability of these data, which were used under license for this study.
Ethics statement Studies using the Clinical Practice Research Datalink (CPRD) are covered by ethics approval, granted by the Trent Multicentre Research Ethics Committee (Reference 05/MRE04/87). CPRD Independent Scientific Advisory Committee approval was granted for this study (ISAC 20‐166).
References
- 1. Tsianakas A, Zeidler C, Ständer S. Prurigo nodularis management. Curr Probl Dermatol 2016; 50:94–101. [DOI] [PubMed] [Google Scholar]
- 2. Zeidler C, Ständer S. The pathogenesis of prurigo nodularis–'super‐itch' in exploration. Eur J Pain 2016; 20:37–40. [DOI] [PubMed] [Google Scholar]
- 3. Mullins TB, Sharma P, Riley CA, Sonthalia S. Prurigo Nodularis. Treasure Island, FL: StatPearls Publishing, 2019. [PubMed] [Google Scholar]
- 4. Whang KA, Le TK, Khanna R et al. Health‐related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol 2022; 86:573–80. [DOI] [PubMed] [Google Scholar]
- 5. Fostini AC, Girolomoni G, Tessari G. Prurigo nodularis: an update on etiopathogenesis and therapy. J Dermatolog Treat 2013; 24:458–62. [DOI] [PubMed] [Google Scholar]
- 6. NORD (National Organization for Rare Disorders) . NIH GARD information: prurigo nodularis. Available at: https://rarediseases.org/gard‐rare‐disease/prurigo‐nodularis/ (last accessed 7 July 2021).
- 7. Genetic and Rare Diseases Information Center. Prurigo nodularis. Available at: https://rarediseases.info.nih.gov/diseases/7480/prurigo‐nodularis (last accessed 7 July 2021).
- 8. Huang AH, Canner JK, Khanna R et al. Real‐world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol 2020; 140:480–3. [DOI] [PubMed] [Google Scholar]
- 9. Ständer S, Augustin M, Berger T et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int 2021; 2:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol 2020; 100:adv00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Misery L, Brenaut E, Torreton E et al. Prevalence and management of chronic nodular prurigo (CNPG) in Brittany (France): estimation by matching two databases. J Eur Acad Dermatol Venereol 2021; 35:e602–4 [DOI] [PubMed] [Google Scholar]
- 12. Ständer S, Ketz M, Kossack N et al. Epidemiology of prurigo nodularis compared with psoriasis in Germany: a claims database analysis. Acta Derm Venereol 2020;14:480–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. CDC National Center for Health Statistics . ICD‐9‐CM guidelines, conversion table, and addenda. Classification of diseases, functioning, and disability. Available at: https://www.cdc.gov/nchs/icd/icd9cm.htm (last accessed 8 October 2021)
- 14. Whang KA, Mahadevan V, Bakhshi PR et al. Prevalence of prurigo nodularis in the United States. J Allergy Clin Immunol Pract 2020; 8:3240–1. [DOI] [PubMed] [Google Scholar]
- 15. International Health Terminology Standards Development Organisation Use SNOMED CT. Available at: www.snomed.org (last accessed 8 July 2021).
- 16. World Health Organization . ICD‐10 version:2016. Available at: http://apps.who.int/classifications/icd10/browse/2016/en (last accessed 8 July 2021).
- 17. Herrett E, Galllagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015; 44:827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hospital Episode Statistics Health and Social Care Information Centre. Available at: http://www.hscic.gov.uk/hes (last accessed 8 October 2021).
- 19. Office for National Statistics . Deaths registration data 2018. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths (last accessed 8 October 2021).
- 20. Benson T. The history of the Read Codes: the inaugural James Read Memorial Lecture 2011. Info Prim Care 2011: 19:173–82. [DOI] [PubMed] [Google Scholar]
- 21. Office for National Statistics . Population estimates for the UK, England and Wales, Scotland and Northern Ireland. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2018 (last accessed 8 October 2021).
- 22. Pereira MP, Basta S, Moore J, Ständer S. Prurigo nodularis: a physician survey to evaluate current perceptions of its classification, clinical experience and unmet need. J Eur Acad Dermatol Venereol 2018; 32:2224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Iking A, Grundmann S, Chatzigeorgakidis E et al. Prurigo as a symptom of atopic and non‐atopic diseases: aetiological survey in a consecutive cohort of 108 patients. J Eur Acad Dermatol Venereol 2013; 27:550–7. [DOI] [PubMed] [Google Scholar]
- 24. Brenaut E, Halvorsen JA, Dalgard FJ et al. The self‐assessed psychological comorbidities of prurigo in European patients: a multicentre study in 13 countries. J Eur Acad Dermatol Venereol 2019; 33:157–62. [DOI] [PubMed] [Google Scholar]
- 25. Singam V, Patel KR, Silverberg JI. Association of prurigo nodularis and lichen simplex chronicus with hospitalization for mental health disorders in US adults. Arch Dermatol Res 2020; 312:587–93. [DOI] [PubMed] [Google Scholar]


