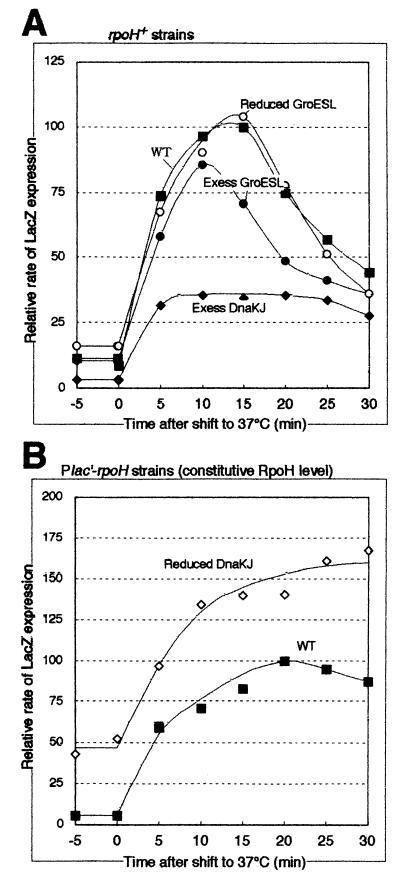
FIG. 7.
Time course of change in RpoH activity (LacZ expression from the pUCD-PrpoH′-lacZ reporter plasmid) during the heat shock response. The same set of strains used in Table 4 were grown in synthetic medium at 25°C and shifted to 37°C at time zero. Samples taken at the times indicated were pulse-labeled with [35S]methionine for 2 min, chased with excess unlabeled methionine for 1 min, and treated with 10% trichloroacetic acid in ice. Radiolabeled E. coli ς32–β-galactosidase fusion protein (GF807-FS [26]) was added as an internal reference to each sample containing the same radioactivity, immunoprecipitated with anti-β-galactosidase antiserum, and resolved by SDS-PAGE (7.5% polyacrylamide). The radioactivities of β-galactosidase bands were normalized to that of the reference protein and are shown as percentages of the maximum synthesis rate in the control (wild-type [WT]) strain. (A) Data for the rpoH+ strain (KN613) carrying pBBR122 (control or WT ▪), pBBR122-dnaKJr (Excess DnaKJ ♦), pBBR122-groESLr (Excess GroESL ●), or KN615 (Ptrc-groE) (Reduced GroESL ○) are shown. (B) Data for the pair of Plac′-rpoH strains, KN209 (control or WT ▪) and KN214 (Reduced DnaKJ ⋄), are shown.