FIGURE 2.
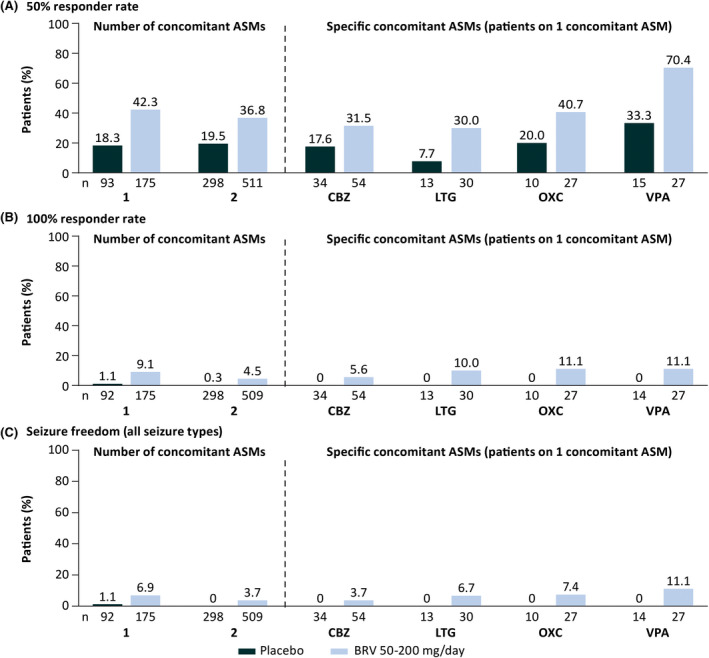
Efficacy outcomes by number of concomitant antiseizure medications (ASMs) and specific concomitant ASMs in patients on one concomitant ASM (patients received placebo or brivaracetam [BRV] in addition to a single concomitant ASM; intent‐to‐treat). (A) Fifty percent responder rate in focal seizures. (B) One hundred percent responder rate in focal seizures. (C) Seizure freedom (all seizure types). Patients were defined as having a 100% responder rate if they had a 100% reduction from baseline in focal seizure frequency. Patients were classified as seizure‐free if they did not report any seizure (any type, including focal seizures, generalized seizures, and unclassified seizures), and had completed their seizure diary for at least 90% of days during the treatment period and did not discontinue during the treatment period. Three patients were excluded from the 100% responder rate and seizure freedom analyses because the only seizures they reported occurred on the first day of the 100% responder rate seizure analysis period, which was 1 day before the seizure freedom analysis period commenced. CBZ, carbamazepine; LTG, lamotrigine; OXC, oxcarbazepine; VPA, valproate.
