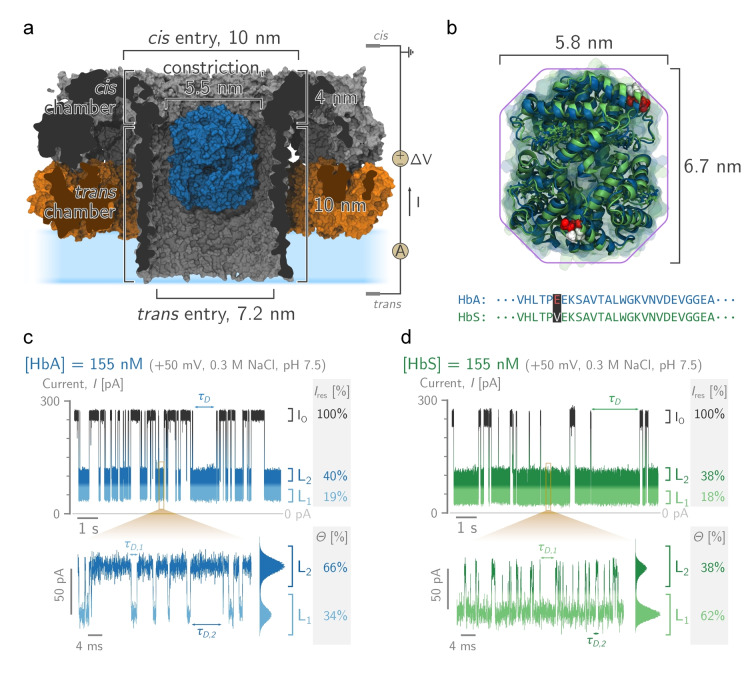
Figure 1.
HbA and S blockades in PlyAB nanopores. a) Molecular surface representation of a single human haemoglobin A (HbA, in blue, PDBID: 2DN1 [47] ) tetramer placed within the trans chamber of a PlyAB nanopore (PlyA and PlyB in orange and grey respectively, PDB ID: 4V2T[ 45 , 46 ]). b) Structural alignment between oxygenated human HbA (in blue, PDB ID: 2DN1 [47] ) and the sickle cell disease HbS (in green, PDBID: 5E83 [48] ). The primary structures of HbA and HbS differ only by a single point mutation in the β‐subunits (E6V, coloured spheres). c) and d) are typical Hb blockades to PlyAB nanopores for HbA (blue) and HbS (green), respectively. In the enlarged trace, the deep and shallow levels are assigned to L1 (light shades) and L2 (dark shades), respectively. A whole trace histogram is shown on the side of the blockade. Hb ( ) is added to the trans side. The recordings were performed at in NaCl, buffered at pH 7.5. Currents were sampled at and filtered using a low‐pass Bessel filter. Molecular structures were rendered using VMD.[ 49 , 50 ]