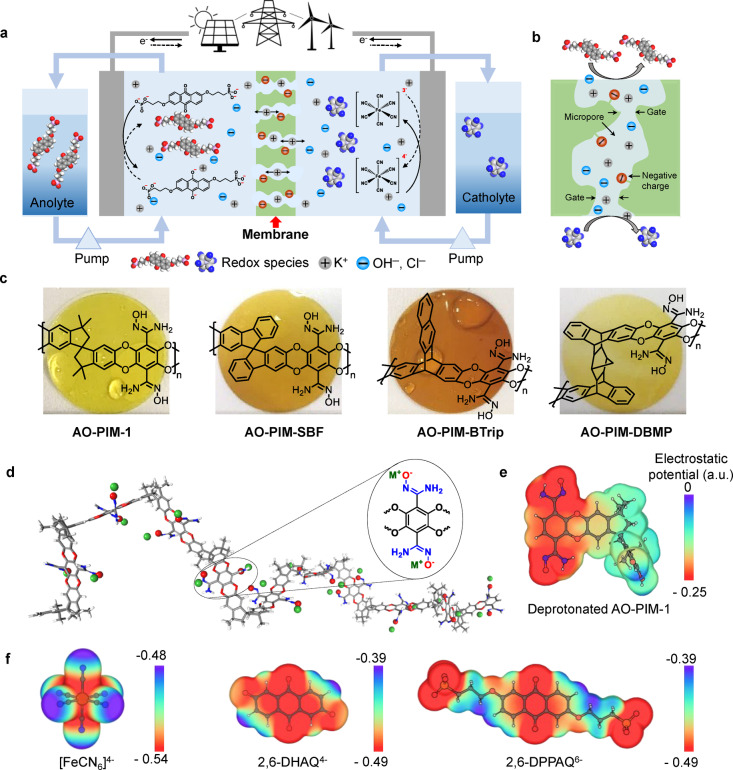
Figure 1.
a) Schematic illustration of an aqueous organic redox flow battery for grid‐scale energy storage. K4Fe(CN)6 and 2,6‐DPPAQ are shown as model redox‐active materials operated at near‐neutral pH electrolytes. Grey and blue balls are described as charge‐carrier ions, and red balls as AO groups, respectively. b) Schematic illustration of interconnected pores with the sub‐nanometre size that enable fast transport of charge‐carrying ions (e.g., K+ and Cl−) while blocking relatively large redox‐active materials. c) Chemical structures and photographs of corresponding 50‐μm‐thick self‐standing membranes of AO‐PIMs including AO‐PIM‐1, AO‐PIM‐SBF, AO‐PIM‐BTrip and AO‐PIM‐DBMP, respectively. The membrane diameter is ≈20 mm. d) Molecular model of AO‐PIM‐1 shows the rigid and contorted PIM backbones with deprotonated AO groups. Electrostatic potential (ESP) of e) AO‐PIM‐1 repeating units and f) redox‐active molecules including [Fe(CN6)]4−, 2,6‐DHAQ4−, and 2,6‐DPPAQ6−.